Healthcare providers, medical device, component and pharmaceutical manufacturers, as well as leading governmental and regulatory agencies, have long recognized the issues healthcare workers face with compounding and administering hazardous drugs, including oncology drugs. These industry leaders have convened to establish policy, increase awareness, and develop specific devices to protect healthcare personnel during occupational handling of hazardous drugs. In December 2019, USP <800> was enacted specifying that CSTDs “should be used when compounding hazardous drugs” and “must be used when administering antineoplastic” drugs when the dosage form allows¹. The development of these regulations and the emphasis on using the appropriate safety devices, such as CSTDs, establish a perspective that the safety of both patients and healthcare workers is significant.
However, various users have revealed a range of potential problems between CSTDs and container closure systems that include device-related issues, such as fluid leakage and broken components to chemical incompatibilities with the devices on the market². These issues elucidate the importance of choosing the most appropriate CSTD to provide physicochemical compatibility with a drug product and ultimately the best protection to the healthcare worker. Additionally, the lack of standard and harmonized performance requirements to demonstrate compatibility between a CSTD and a drug container closure system further complicates the issue. In response, working groups like Product Quality Research Institute (PQRI) are developing test standards and guidance for evaluating the inter-connectability between the vial and transfer device³.
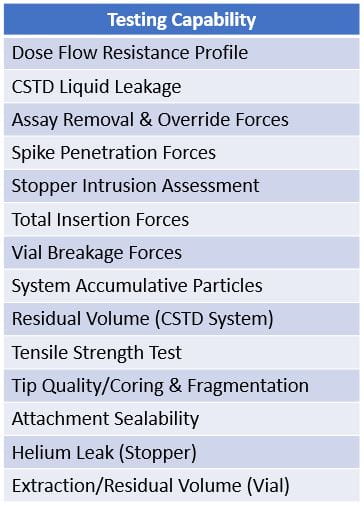
Our expert team of dedicated scientists in West’s Analytical Services and Scientific Insights Labs have partnered with several organizations to establish multiple CSTD test capabilities and methods to investigate the issues with the container closure component or interfaces with the CSTD. Capabilities include the following:
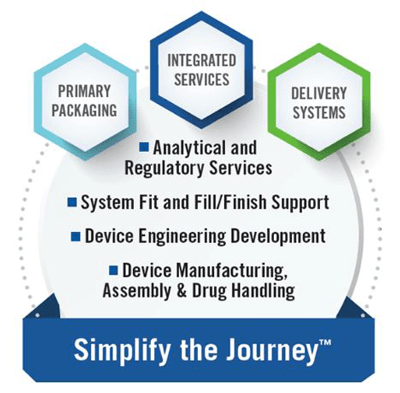
West is “By Your Side” as you navigate the challenges of assessing a CSTD with your drug product container closure system. Please visit our Analytical Labs page to learn more about our expertise and testing capabilities.
Reference:
- USP [2016]. USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Setting. Rockville, MD: United States Pharmacopoeia, accessed USP [2016].
- FDA Manufacturer and User Facility Device Experience (MAUDE) Database, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm, accessed 12Oct2020
- Cathy Zhao and Allison Radwick; 5 Challenges of Closed System Transfer Devices Parental Drug Association letter, Manufacturing Science 07Jan, 2020
















)
)





