Daikyo Crystal Zenith® (CZ) Ready-to-use Nested Vial in Tub: Advantages of New Design and Packaging Configuration of Crystal Zenith® Vials for Cell and Gene Therapy Drugs
Cell and gene therapies (CGTs) are becoming therapeutic modalities of choice for complex diseases like cancer, autoimmune and genetic disorders etc. In cell therapies, the cells are sourced either from the patient or a healthy donor and are genetically modified to fight diverse disease conditions within the body, whereas in gene therapies, malfunctioning genes in the cells are replaced with a working copy of the gene. With the advent of these advanced therapies, biopharma industry is facing many challenges not only in developing molecules against new hard to target conditions but also in the logistics of scaled up manufacturing. These therapies were in early stages during the past years and logistics of small batches that are often required for clinical trials were sorted out. As these therapies are becoming mainstream, scaling up or scaling out is becoming a challenge to reach more patients mostly due to intricacies of the manufacturing and fill/finish process that differs from conventional drugs. Gene therapy drug products are mostly filled in a traditional manner within an isolator in vials whereas for cell therapies closed processing and filling in either cryo-bags or vials is the current norm. However, as more therapies are getting approved along with the advent of allogenic cell therapies that will have relatively large batch sizes, more innovation, and new products are required for fast packaging needs as they tend to become less efficacious with increased processing times due to their sensitive nature.

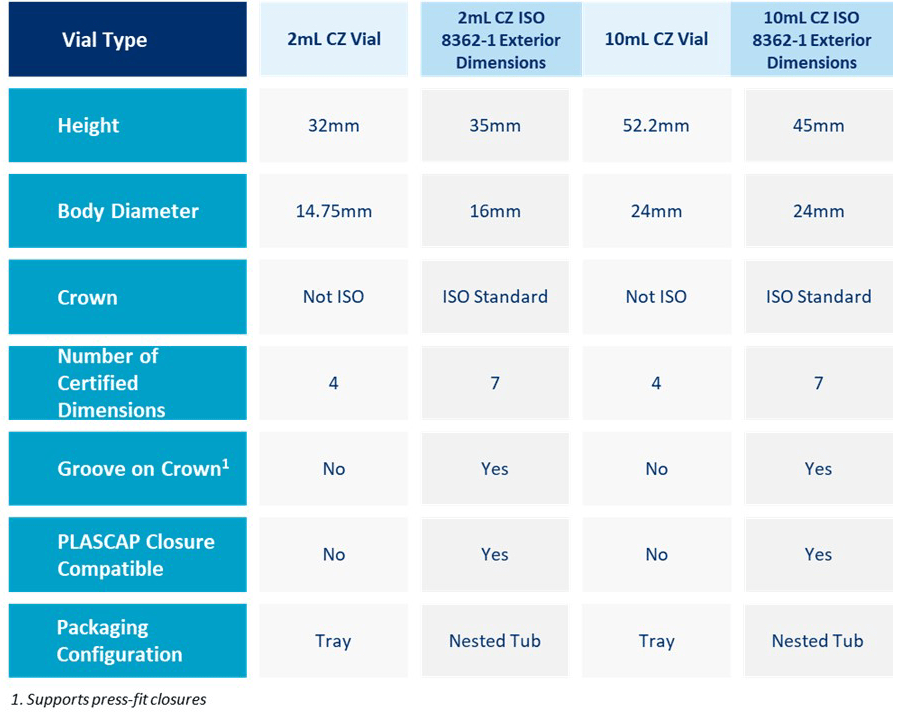
CGTs are still an emerging field with regulatory guidelines becoming more mature for different aspects of drug development including primary packaging with each passing approval. To accelerate the time to the market and to overcome regulatory hurdles it is important to adopt market leading containment solutions that have already been approved for several similar drug products. West Pharmaceutical Services, Inc., a leader in providing containment solutions for different classes of drug products, is now providing Daikyo Crystal Zenith® (CZ) nested vials in tub designed to meet selected exterior dimensions for ISO glass standard 8362-1. These vials are made up of high-quality cyclic olefin polymer (COP) which is proven for packaging advanced therapeutics such as gene therapy. It offers reduced risk of breakage over glass but with similar transparency and protein recovery along with no ion or (heavy) metal release that ensures drug safety, purity, and efficacy. Reduced delamination risk of CZ vial material and the best-in-class particle specification of CZ nested vials (2% according to USP <788> compared to 10% industry standard) along with ISO exterior dimensions makes it a preferred primary packaging solution1. Along with previously performed studies on CZ vials that shows higher viral vector recovery than glass or polypropylene vials2 and its ability to maintain container closure integrity (CCI) at both ultra cold (-80 °C) and cryogenic temperatures (-196 °C) when used along with NovaPure® stoppers and Flip-Off® Clean Certified Sterilized (CCS) seals3 makes it highly suitable for advanced therapeutic drug products. In fact, multiple approved gene therapy drug products utilize CZ vials as primary container demonstrating its compatibility with these high value, sensitive drug products and meeting the rigorous performance requirements. The new CZ nested vials meet the ISO 8362-1 standard exterior dimensions and are available in 2 mL and 10 mL sizes. The nested packaging configuration is compatible with the industry expectations outlined in ISO 11040-7 and ISO 21882 (Fig.1 & Table 1). Additionally, the packaging system has been evaluated by multiple fill/finish equipment manufacturers and offers broad compatibility with various fill/finish solutions that are available in the market.

Fig. 1: 2 mL and 10 mL CZ Vials in Nested Configuration
Table 1: Vial Specification of CZ vials and CZ Nested Vials designed to meet ISO Exterior Dimensions
Time and ease to fill/-finish is a critical factor for advanced therapy drug products, especially in the case of cell therapy, as the presence of the cryoprotectant DMSO in the final formulation can be detrimental for cell viability. CZ nested vials will accelerate the fill/-finish process by reducing the processing time as it follows a conventional nest and tub packaging configuration and hence is broadly compatible with various fill/finish equipment in market. The rigid structure of the tub protects vials during transit, preventing unwanted breakage and improves machinability for fill/finish equipment. The 2 mL CZ nested vials consist of 100 vials in 10X10 format whereas the 10 mL CZ nested vials consist of 48 vials in 6X8 format which are consistent with the currently available nested glass vials. The bottom of the nest comprises of an opening underneath each vial pocket that allows individual lifting of vials to facilitate desired operations like filling, crimping etc. CZ nested vials are sealed with a breathable lid and packaged inside a non-breathable inner bag and breathable outer bag. The lid and the inner bag both serve as sterile barriers that can be removed either manually, semi-automatically or with automated systems based on customer preferences and equipment availability. CZ nested vials come in a clean, sterilized, Ready-to-use (RU) format that aligns with Annex 1’s requirement to prevent contamination in the final product (Fig.2) and is available in a configuration of 6 tubs per carton. For vial closure CZ nested vials offer flexible stoppering solutions with NovaPure® stoppers paired with Flip-Off® CCS seals (Fig.3A) or with Daikyo PLASCAP® Ready-To-Use, Validated closure solution which is an integrated stopper-and-seal/cap that enables stoppering and sealing in a single fill/finish operation (Fig.3B) to meet manual to semi-automated or automated filling requirements. The availability of PLASCAP in a nested format (Fig. 3C) further makes it easier to use with CZ nested vials during the fill/finish process as the stoppering and sealing can be performed for the entire nest of vials in a single operation.
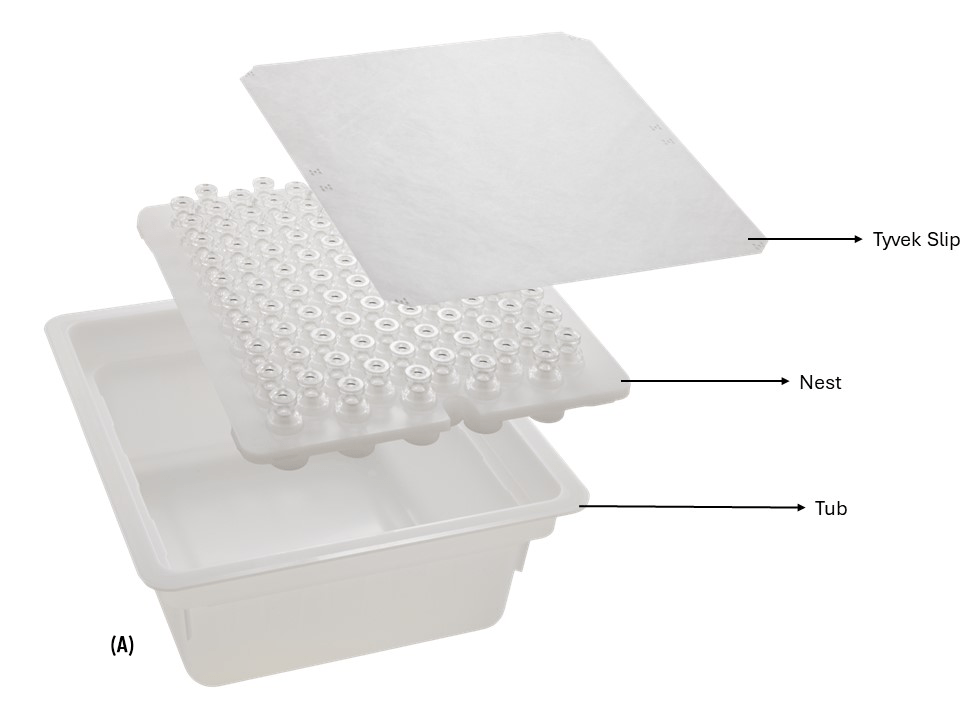
Fig.2: (A) Representative image of CZ nested vials showing the Tyvek slip, Nest and the Tub
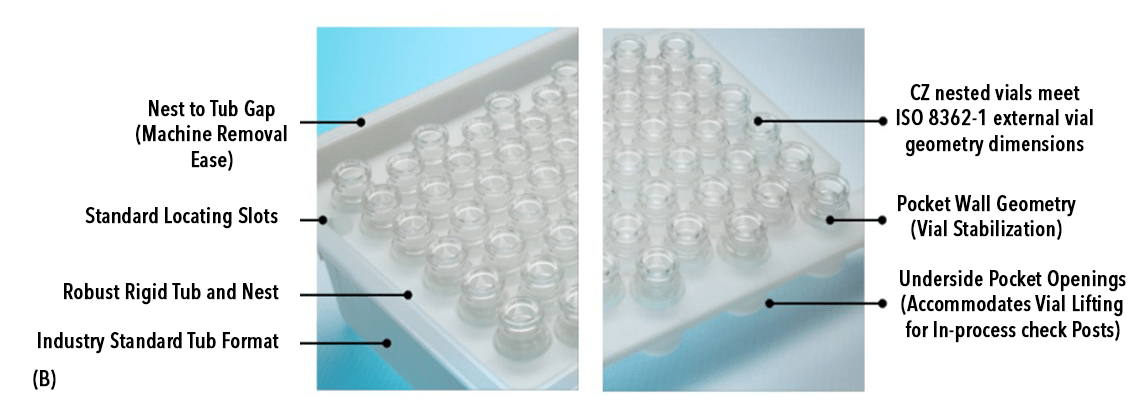
Fig.2: (B) Salient features of the nest and tub that helps in fill/finish operation.

Fig.3: (A) NovaPure® stopper with Flip-Off® CCS seals (clean, certified, sterilized) (B) 13 mm and 20 mm PLASCAP with integrated stopper, (C) PLASCAP nest compatible with CZ nested vials
The CZ nested vials with exterior dimensions according to ISO 8362-1 provide great flexibility, as they are suitable not only for CGT drug products but can be used for other drug products as well. Overall, the West pharmaceutical services products mentioned here offer a convenient and efficient packaging solution for small batch fill/finish operations for advanced therapeutics.,
Crystal Zenith and PLASCAP are registered trademarks of Daikyo Seiko, Ltd. and are used under license from Daikyo Seiko.NovaPure and Flip Off are registered trademarks of West Pharmaceutical Services, Inc. In the United States and other jurisdictions.
References:
- Data available internally.
- Functional assessment of AAV (Adeno Associated Virus) stored in Daikyo Crystal Zenith® vials at Ultracold temperatures (TR 2021/033)
- Performance of Daikyo Crystal Zenith® 2 ml vials at cryogenic temperatures: container closure integrity and cell preservation








