Eyeing the Future by Innovating in Ophthalmic Drug Delivery
In the intricate landscape of healthcare, ophthalmology stands out as a domain where innovation is not merely encouraged – it is imperative. As we delve into the realm of eye health, the question looms How can the pharma and medical device industry support patients journey? By exploring the vast unmet need in ophthalmology and focusing on developing advanced drug delivery systems, enhancing patient adherence through less invasive treatments, and improving biocompatibility and efficacy of ophthalmic devices, the industry can lead the way in this critical field and significantly ease patients’ journey.
The Looming Burden of Vision Loss
As the world ages, the prevalence of eye diseases such as age-related macular degeneration (AMD), geographic atrophy and diabetic retinopathy is rising significantly. By 2040, age-related macular degeneration will affect 288 million patients worldwide (1). By 2030, ~14 million patients will be treated with intravitreal injections for retinal diseases. With a patient receiving ~ 4-8 injections/ year, this translates to ~ 112 million injections/year (2) . Without proper adherence to these maintenance injections, patients face the risk of irreversible vision loss and thereby their independence.
 Vision of a Patient Suffering from Advanced Stage of Age-related Macular Degeneration
Vision of a Patient Suffering from Advanced Stage of Age-related Macular Degeneration The Patient Experience: Understanding the Need Through Empathy
To truly innovate, we, medical device companies must step into the shoes of our patients. For a patient navigating through the complexities of eye care, particularly in a retina clinic, the path is fraught with uncertainty, discomfort, and the threat of vision loss looming, patients face difficult and uncomfortable decisions. An average AMD patient is >70 years of age. Outside of getting the injection the patient must deal with the journey to the clinic, long wait times, monitoring of the injection, and dependency on caregivers for making the trip to the clinic and back. A large majority of these patients have an advanced disease state, which makes them legally unfit to drive. The pain and the discomfort generally last two to three days. To repeat this process every 4 weeks, is indeed a huge burden on the patients and their caregivers. Moreover, the regimens are fraught with poor compliance with the patient missing on the full benefits of the treatment.
Now imagine an elderly woman, Ethel, in her seventies with late-stage Age related Macular Degeneration (AMD). Having travelled to meet a friend, she is waiting at the bus station for her friend to pick her up. She suffers from poor vision and has forgotten her friend’s address who she came to visit. Her non-smart phone is piling up with missed calls from her friend. She is stressed and helpless. Her treatment story- She refused to go in every month to get an injection due to the huge toll it was taking on her physically and emotionally. She did not live with any kin. Her story signifies the loss of independence and poor quality of life that patients with retinal diseases experience daily. Fortunately, for her, other commuters at the bus station were able to track down her friend who was able to pick her up. Unfortunately, this is not an isolated incident.

An Older Woman with Deteriorating Vision at a Bus Station Recently, ophthalmology – and retinal delivery is shifting towards treat-and-extend regimens, with emerging biologics extending durability to once every 6 months after an initial loading dose injection. This is a welcome change for patients and can significantly help improve patient compliance and therapeutic benefit. The port delivery system – where a depot drug chamber implanted into the patients’ eyes is refilled with the drug periodically is an example where durability can be extended beyond 6 months.
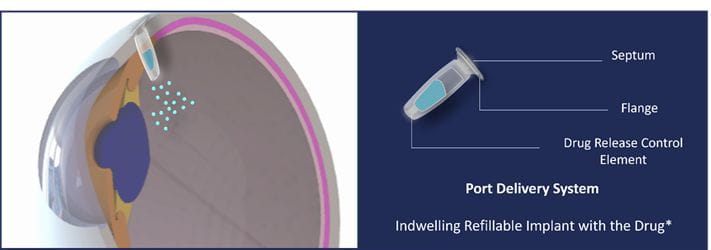
Port Delivery System (PDS), an indwelling implant device engineered to continuously deliver a customized formulation of ranibizumab drug for maintaining therapeutic drug concentration. The PDS is filled with a drug using a custom needle and it provides continuous drug delivery into the vitreous (clear, colorless, jelly-like substance that fills the posterior chamber of the eyeball) by passive diffusion. With just two refills per year, PDS significantly reduces the burden of frequent injections and physician visits (3). PDS implant with Ranibizumab drug is an approved product of Genentech Under the brand name Susvimo®. *This image is generated by the author for depiction purposes only.
Where Does Containment and Delivery Solutions Fit in This Patient Journey?
Solutions such as the port delivery system offer significant therapeutic benefit of extending the durability of the therapy– however, it is still a surgical procedure performed in an operating room that has limitations in cost, coverage, risk of infection and complications arising from septum dislodgement. However, recent relaunch has since updated the ocular implant and refill needle, as well as improved the manufacturing process (4).Innovation in this space is looking for easy to implement technologies that enable care to be done more effectively in a retina clinic.
Delivery Solutions for Long Acting Injectables
Some of the more emerging strategies of improving durability require innovations in delivery technologies that can integrate seamlessly in a clinical workflow in a retina clinic. For instance, drugs can be made more durable by concentrating them. In the case of biological monoclonal antibody (mabs) drugs such as anti-VEGFs, this poses unique challenges pertaining to protein stabilization, and the ability to deliver the drugs due to increased viscosity. Looking ahead drug delivery device innovation can also cater to providing containment and delivery solutions for emerging sustained-release formulations or extended-release drugs which are inherently viscous. These sustained-release formulations such as hydrogels, microparticles, rods, or bioconjugated polymers, extend the durability of therapies by slowing the release of the active component, thereby maintaining the minimum effective concentration in the ocular tissue compartments for an extended period. Due to their high viscosity and tendency to sediment or other physicochemical properties unique to the presentation of this formulation, these sustained release technologies require expertise in air-bubble free fill finish, novel compatibility assessments, and delivery or deployment technologies.
Innovative Delivery Technologies for Newer Routes of Administration
Since the approval of Ranibizumab (Lucentis) in 2014, intravitreal delivery has emerged as the most common in-office procedure for drug delivery, with 10M intravitreal injections performed annually in the US (5). However, the intravitreal space is not suitable for emerging drugs such as gene therapies due to the risks of inflammation caused from leakage into the systemic circulation. Further, it is essential for the drug to be delivered in the inner retina , where the capsid needs to cross various restrictive biological membrane barriers to reach the target site, which significantly limits the therapeutic efficiency. Although sub-retinal injections allow direct delivery into the retina, this remains a highly invasive procedure involving vitrectomy surgery and a complicated injection into the sub-retinal membrane using a 38G cannula. This surgical procedure demands extreme dexterity and precision. Moreover, it is expensive and cannot be democratized for millions affected by vision-threatening conditions under the current infrastructure.
Although sub-retinal injections allow direct delivery into the retina, this remains a highly invasive procedureinvolving vitrectomy surgery and a complicated injection into the sub-retinal membrane using a 38G cannula. Thissurgical procedure demands extreme dexterity and precision. Moreover, it is expensive and cannot be democratizedfor millions affected by vision-threatening conditions under the current infrastructure.
Suprachoroidal Delivery has emerged as a “best of both worlds” offering targeted delivery with the added convenience of an in-office procedure. Targeted delivery is achieved by injecting the drug into a potential space between the sclera and the choroid which allows the drug to flow posteriorly and circumferentially to the retina in the back of the eye. However, this is an emerging route with only one approved drug currently available for administration. Gene therapies and several other small molecule targets are in the pipeline for this method of delivery. There is significant opportunity for streamlining and improving this injection through improved tissue access, physician training, and patient education. There is opportunity to explore this novel route of administration to improve the durability of therapies, particularly those with longer residence times, such as sustained release and particle-based drug delivery systems.
One and Done Promise of Gene Therapy- Currently there are more than 129 ocular gene therapy products in various stages of clinical trials, of which 87 programs treat macular degeneration (40 Wet AMD & 29 Dry AMD) (6). Gene therapies such as protein biofactory based approaches have the promise of one and done for sustained therapy, particularly for acquired retinal diseases. Administration of viral vector-based gene therapies can convert the eye into an “ocular biofactory” where the eye now produces non-native proteins that have beneficial properties. Gene therapy strategies focusing on retinal cells to produce anti-VEGF proteins intraocularly in sustainable amounts have proven to be attractive avenues for treatment of Wet Age-Related Macular Degeneration (Wet AMD). Theoretically, a successful single treatment can turn the eye into a biofactory to produce the anti-VEGF protein for the lifetime. Despite the convenience and promise, these therapies are still in trials, and their lifetime durability remains to be seen. In the best case, gene therapies will coexist with treat-and-extend regimens, with patients having hard to control cases being treated with gene therapy.
Reducing Treatment Costs by Decreasing Drug Wastage and Improving Precision in Delivery
US retina specialists perform 2.5 million injections/year today, with busy retina specialists performing as many as 50 per day. $12-18 Billion/year is lost in estimated drug wastage due to 70% overfill of anti-VEGF therapies (7). The cost of drug wastage to gene therapies can be even more substantial considering the high cost/dose.
Precision is therefore another area where innovations in delivery technologies can significantly improve the patient outcome. Because the eye is a closed compartment injecting an excess than the prescribed 0.05 mL volume referenced previously often results in a transient increase in intraocular pressure (IOP), potentially leading to vision-threatening damage to the optic nerve. Improving precision of delivery devices can eliminate the issue of inadvertent increase in injection volumes.
So, the natural question for us to ask is – Where can the drug delivery systems industry make the biggest difference for people like Ethel? With growing number of diseases requiring continuous maintenance injection, the biggest impact is transforming the durability of current and future therapies, thereby minimizing the burden of patient care. The impact of drug delivery systems in releasing medication over extended periods—stands as a cornerstone of this transformation. The ability to deliver treatments that last six months or longer not only optimizes overall therapeutic efficacy but also significantly enhances patient experience, giving then the control they deserve. With their whole life being planned around monthly injections, better delivery technologies give the patients better visual outcomes and a life they deserve.
References
- Wong, Wan Ling et al. Global prevalence of age-related macular degeneration and disease burden projection for2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health, 2014; 2(2).
- Mathew W. MacCumber, MD, PhD, Real-world injection intervals in Wet AMD, Retina Today. May/June 2020/ SpecialReport.
- Roche Euretina 2023 Presentation - The future of continuous drug delivery for retinal diseases.
- Genentech to reintroduce Susvimo for people with wet age-related macular degeneration (AMD). Genentech.Businesswire.com, July 8, 2024.
- Wang R, et al. Quantifying burden of intravitreal injections: questionnaire assessment of life impact oftreatment by intravitreal injections (QUALITII). BMJ Open Ophthalmol., 2022;7(1)
- Ameri H, Kesavamoorthy N, Bruce DN. Frequency and Pattern of Worldwide Ocular Gene Therapy Clinical Trialsup to 2022. Biomedicines, 2023;11(12).
- ASRS survey 2020.







