Leachables Method Development and Validation and the Updated Relevant ICH References Part 3 – Method Validation
This is part three of a three-part series about how recent updates to ICH Q2 and Q14 can be applied to the development and validation of leachables methods. Check out part one focused on the background and history of the updated ICH references and two focused on method development.

Now that our new leachables method has been developed following the new ICH Q14 guideline, it should be validated per the ICH Q2(R2) guideline on validation of analytical procedures. The validation procedures for chromatography and mass spectrometry methods are often similar from method to method. However, it should still be a best practice to review this guideline and apply it systematically where possible.
As in previous versions of this guideline, it shows the typical validation tests to be performed based on the type of method that is being validated. Considering that leachables are a type of impurity, albeit often at trace concentrations, this puts leachables methods through the most rigor described in the guideline. This includes tests to demonstrate specificity, range, quantitation limit, accuracy, and precision, which has not changed from the previous guideline. However, there are some notable additions to some of these that are worth mentioning when going through them systematically with this example method.
To test for specificity, the most appropriate approach for our GC/MS method would be to perform a test that shows the absence of interference which would affect the identification and quantitation of the target leachables. This can be done by testing aliquots of the sample matrix spiked with the target leachables and comparing to sample matrix without these targets added. However, the guidance does offer the possibility to use inherent technical aspects to justify specificity. If a high-resolution mass spectrometer were employed to target very specific ions, a specificity lab test may not be needed.
Like specificity, the fundamentals of determining the range of the method have not changed. It still represents the concentrations from lowest to highest for which the method provides accurate results. In the new guideline, however, the calibration range is no longer referred to as just “linearity” and instead it is called “response,” which now includes descriptions of non-linear response and multivariate calibrations. This is a useful addition for leachables methods since there may be occasions where a leachables analyte does not respond linearly, especially in mass selective detectors. It should be determined during development how the various leachables targets respond and to define and specify their types of responses for the validation test, with the criterion being able to meet a certain coefficient of determination (R2). In our method the calibration of each target leachable can be different if needed as long as their predictability is demonstrated during validation.
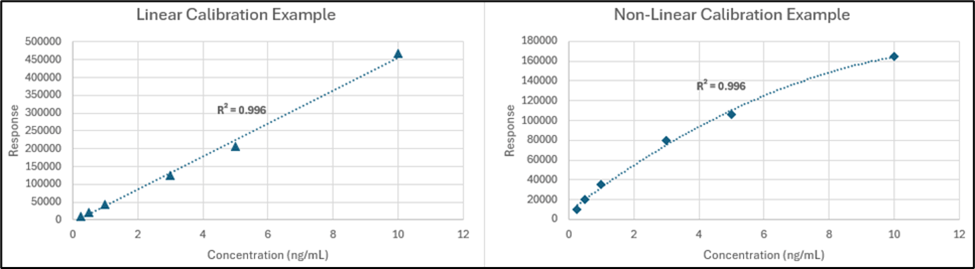
Figure 1. Examples of possible linear and non-linear calibrations
Within the topic of range is the validation of lower range quantitation limits and detection limits (“QL” and “DL,” respectively). Recall that the lowest concentration for which the method can give an accurate result is the lower range of the method. Typically, a test is performed to demonstrate detectability at the desired QL, which in a leachables method should be lower than the analytical evaluation threshold (AET) of the target leachables. A DL does not need to be defined for an impurities method. The guideline describes the commonly used signal-to-noise ratio (S/N) approach where the analyte shows S/N at the desired concentration at or above 10, which may be appropriate in our method if we use the mass spectrometer in scan mode and generate a lot of noise. With the prevalence of data deconvolution techniques and high-resolution mass spectrometers; however, what is used for quantitation may be just a single ion. This may result in no noise to measure, making a signal-to-noise calculation meaningless. In these cases, the use of accuracy and precision results at the QL can be used as well as alternative statistical approaches.
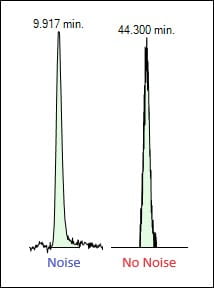
Figure 2. Examples of a signal with noise and a signal without noise
The guideline for accuracy and precision characteristics and tests have not changed with the new revision. They can still be evaluated in combination (which is always recommended for a laboratory that wants to validate efficiently); accuracy should still cover the entire range of the method and have at least nine measurements; precision can still be done with six measurements, which should be done at the AET. Intermediate precision is where there is a small change in the guideline. It now references the method’s development and risk assessment per the new ICH Q14 guideline to help an operator determine the extent of the intermediate precision test. Typical variations may include different days, analysts, and/or equipment, but the choices made should be relevant in the context of the method’s use.
Overall, the update to ICH Q2(R2) and the new Q14 guideline contain familiar concepts, but also welcome and helpful changes to accommodate more modern technology. These guidelines can be applied to leachables methods, along with all the other types of methods that are explicitly stated as being in scope, to ensure that methods are developed and validated thoughtfully and will perform well over an entire leachables stability study.
For more information on our Extractable and Leachable expertise and testing capabilities click here.





