A robust extractables data package helps drive patient safety
Focus on extractables and leachables (E&L) from packaging and delivery systems began in the mid-1990s but, since then, regulatory agencies have become more educated, and expectations of the pharmaceutical industry have increased. Examples of updated recommendations include USP<1663> and <1664> and injectable drug recommendations from Product Quality Research Institute (PQRI). Complex drug product formulations have become more common, so it is imperative to have a deeper knowledge of extractables to understand risks associated with potential interactions between packaging and drug product.

To manage these risks , where should you start?
The most important step is to understand the extractables from the packaging components, since there are chemicals which may leach during normal storage and use. However, generating extractables data can be time consuming if a new extractables study is needed. One way that this can be mitigated is to have robust extractables data of the materials of construction from your packaging supplier. There are other risk factors which can affect leaching, such as storage conditions, drug matrix, and dosing regimen, but without knowing what the extractables of your materials are, there is not a good way to trace leachables back to a material source.
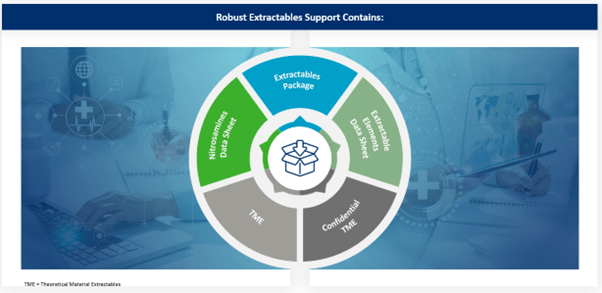
To assist customers in developing a comprehensive, risk-based approach to the evaluation of extractables and leachables, West has developed a robust Materials Characterization extractables data package for elastomer 4031/45 Gray, which is available to help customers truncate extractables work. Four critical areas were assessed:
- Pre-Planning and Component Selection
- Gap Assessment and Evaluation
- Risk Assessment
- Leachables Evaluation
In addition, West also provides data packages regarding nitrosamines and extractable elements. Together with the robust extractables data package, these give customers the knowledge they need to fully understand potential chemical safety risks that patients could be exposed to, enabling them to make better risk-based decisions when deciding which components will be part of their primary packaging solution.
The analytical data in the extractables package was obtained in West’s GMP laboratory in Exton, PA. To provide a comprehensive evaluation, Westar™ Ready-to-Use quality level elastomer products were tested using multiple analytical techniques. The 4031/45 Gray robust extractables data package can be attained by contacting West Technical Customer Support via our Contact Us form.
Additionally, West offers the assistance of its scientific staff for consultation through the component evaluation process, and its laboratories for execution of related studies. The robust extractables package, along with West’s knowledge in extractables and leachables testing, make West a great choice for customers who must make data-driven and risk-based decisions in consideration of extractables and leachables. For further details on your timeline for planning E&L assessment take a look at our whitepaper, Is it Ever Too Soon To Start Your Extractables and Leachables Assessment?
Westar is a trademark of West Pharmaceutical Services, Inc. in the United States and other jurisdictions.




