How can Westar™ RU and Westar™ Select RU quality level components address sterilization issues?
Sterilization processes are fundamental and critical in the manufacturing of sterile pharmaceutical products. According to the US Code of Federal Regulations, “Drug product containers and closures shall be clean and, where indicated by the nature of the drug, sterilized and processed to remove pyrogenic properties to assure that they are suitable for their intended use."1 When considering elastomeric components, common methods for sterilizing include steam and gamma. This is where the sterilized components with Westar™ Ready to use (RU) and Westar™ Select RU quality levels come in. Westar™ RU and Westar™ Select RU quality level products are supplied ready to be used and help simplify and speed up the drug manufacturing process while maintaining high standards of quality and safety.
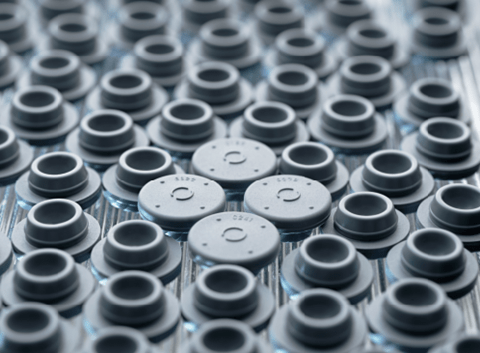
Common Types of Sterilization
Sterilization is a process whereby all forms of microbial life are inactivated via physical or chemical methods. Some of the common types of sterilization, are defined below along with potential challenges associated with each method.
Steam sterilization is a widely used sterilization method which involves exposing the product to high-pressure steam for a specified duration. While it is an effective method of sterilization, it requires high capital costs for the equipment, significant energy consumption.
Gamma irradiation is also a popular sterilization method that involves exposing the product to ionizing radiation. This method is useful for products that are sensitive to heat and moisture and does not require the use of chemicals (other than the starting material and by-products of the gamma irradiation process). However, it requires specialized equipment and facilities and can cause changes in the physical and chemical properties of the product.
Evidently, there is not a one-size-fits-all solution when it comes to sterilization. Each method has its limitations and even so, there are many other concerns with regards to product efficacy, stability, and overall quality. Fortunately, Westar™ RU and Westar™ Select RU quality level products can eliminate many of the concerns that customers have, so that they can focus on manufacturing efficiency.
What are Westar™ RU and Westar™ Select RU?
Elastomeric components with Westar™ RU and Westar™ Select RU quality levels have undergone a validated pharmaceutical wash and sterilization processes, to supply RU components ready for direct introduction into sterile fill finish operations. After the pharmaceutical wash process, the components are loaded into packaging designed to maintain product sterility after sterilization, throughout its lifecycle, including transportation and storage.
By using Westar™ RU and Westar™ Select RU quality level components, benefits are transferred to customers, such as reduction in capital investments for component washing, siliconization and sterilization, and enhanced risk mitigation and flexibility with multi-site global manufacturing capabilities. As a result, less manufacturing footprint and validation work is required, and lower risk is incurred by the customer, as compared to having the sterilization performed in-house.
Features of Westar™ RU and Westar™ Select RU quality levels
Processing of the Westar™ RU and Westar™ Select RU quality level components comprises two steps. First, the elastomeric components undergo the West global pharmaceutical wash process, which is validated for reduction in bacterial endotoxin by at least 99.9% (3 log reduction). Additionally, the release specification for this wash process includes particulate matter and bioburden. Siliconization of components can also be included during this process, if applicable. These parameters are tested for every batch and reported on the quality certificate, which are supplied together with the shipment.
Following the pharmaceutical wash process, the elastomeric components undergo either the pharmaceutical steam sterilization process or pharmaceutical gamma sterilization process, both validated to provide a minimum sterility assurance level (SAL) of 10-6. This means that there is less than or equal to one in a million chance that a component remains non-sterile. Additionally, these processes, together with the material and equipment involved, are continuously maintained with revalidation and periodic review programs to comply with the relevant current Good Manufacturing Practice (cGMP) requirements and various international regulatory standards. This provides quality assurance to customers that the product is free from viable microorganisms and is safe to use.
The main difference between components with Westar™ RU and Westar™ Select RU quality levels is that the latter undergoes an optimized wash program, thus allowing tighter specifications for particulates > 100 µm, Proved Clean Index (PCI) and an enhanced acceptable quality level (AQL) program.
Besides the tests mentioned above, the sterilized elastomeric components are also verified to meet compendia, physical and functional specifications post sterilization.
Why choose Westar™ RU and Westar™ Select RU quality
West recommends steam sterilization over high-dose gamma irradiation as risks from chemical and physical deterioration of elastomers can be mitigated. This is performed through an optimized steam sterilization cycle, which entails:
- Ensuring the integrity of the elastomer’s sterility, physical and chemical properties through its shelf-life
- Minimize the extractables and leachables between the elastomer and the drug product
- Improved component functionality
By tapping into West’s expertise of Westar™ RU and Westar™ Select RU quality levels, customers can be reassured that the product meets the necessary compliance and compendial requirements. As such, the customer benefits from reduction of testing, validation studies, labor and maintenance costs. The validated process information is filed in the Drug Master File (DMF) under US Food and Drug Administration (FDA), ready to support regulatory review with issuance of the Letter of Authorization (LOA) from West upon request.
All in all, Westar™ RU and Westar™ Select RU quality level products can help eliminate the need for specialized equipment and facilities, whereby customers can focus on their core business activities, and reduce their capital and operating cost. West pharmaceutical wash and sterilization processes are extensively validated to ensure that the products are washed and sterilized to high standards, providing peace of mind for customers and patients alike. For more details on Westar™ RU and Westar™ Select RU quality level offerings, click here or contact an Account Manager or Technical Customer Support (TCS) representative.
References
1 United States Food and Drug Administration. (2023). PART 211 -- CURRENT GOOD MANUFACTURING PRACTICE FOR FINISHED PHARMACEUTICALS Subpart E - Control of Components and Drug Product Containers and Closures, Sec. 211.94 Drug product containers and closures. (Accessed on 18 Aug 2023)https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=211.94
Westar™ is a trademark of West Pharmaceutical Services, Inc. in the United States and other jurisdictions.














