Plan for Your Extractables and Leachables Studies to Meet your Submission Timelines
Demonstrating the compatibility of any material in contact with drug product throughout its lifecycle (manufacture, containment, and delivery) is a necessity for a regulatory submission. Defined as extractables and leachables, these assessments continue to evolve and expand due to consistently increasing regulatory agency expectations. Agency expectations require you to act sooner rather than later. Waiting until Phase III in drug development can lead to delays and additional costs due to poor assessments that are questioned by the reviewer, or incomplete submissions that require you to redo or execute additional work. You can start your Extractables and Leachables (E&L) assessment as soon as you have selected your packaging components.

E&L assessments are multi-step processes where you must use knowledge of your drug product and manufacturing process, along with data from vendors, analytical labs, and toxicologists to demonstrate compatibility between the drug product and any material in contact with the drug product. Compatibility, in the framework of E&L, means that no compound will migrate from the material and have a significant negative impact on the drug product or the patient. Guidance documents from the United States Pharmacopeia (<1663>, <1664>) and the Product Quality Research Institute (Safety Thresholds and Best Demonstrated Practices for Extractables and Leachables in Parenteral Drug Products [Intravenous, Subcutaneous, and Intramuscular]), and ISO standard 10993-18 provide a framework of how to execute this assessment. The framework is as follows:
- Step 1 - Educated Component Selection
o Use established materials from reputable vendors
o Review initial materials provided by the vendors
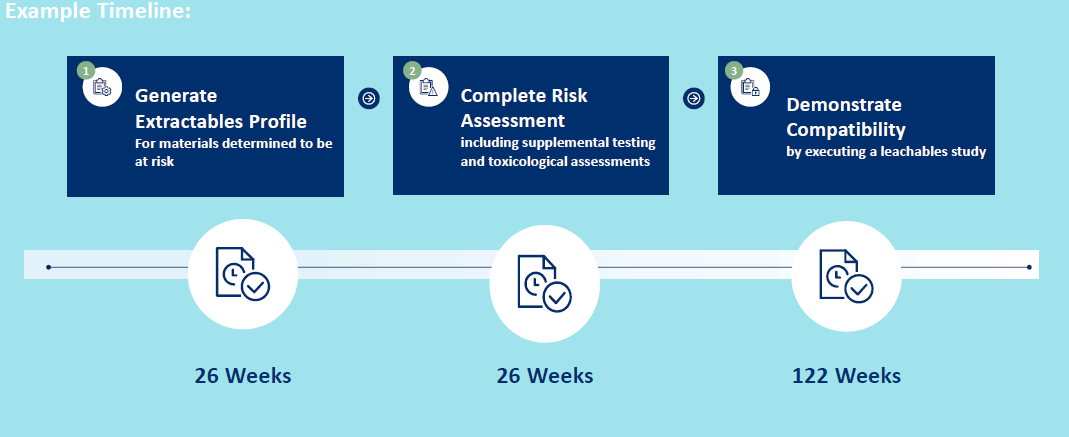
- Step 2 - Generate Extractables Profile
o Ascertain compounds that could migrate from components – or extractable compounds
o Evaluate the risk to the patient and the product associated with the compounds that comprise the extractables profile.
- Step 4 - Demonstrate Efficacy and Safety
o Execute leachables study
Step 1 - Educated Component Selection
You must first gather pertinent data around the materials of use and the drug product such as drug contact time, temperature, and proximity to final product for processing materials; storage conditions and desired shelf life for primary packaging components; and pH range and constituents of the drug product.This data will aid you in planning and executing an extractables study. For example, West can provide Theoretical Material Extractable lists (TMEs) and Material Characterization documents and even provides VeriSure® Technical Packages for West’s leading elastomer formulations.
Step 2 - Generate Extractables Profile
After evaluating this data collected in step 1, an extractables assessment is designed using applicable guidance documents (examples include USP <1663> for packaging materials, USP <665> for polymeric manufacturing components, ISO10993-18 for medical devices or delivery components on a combination product) to stress packaging materials using exaggerated conditions to understand what could leach into your drug product under real work conditions.The execution of an extractables study typically involves the following steps:
- Extraction of the materials
- Preparation and analysis of resulting extracts
- Data interpretation
- Reviewing and ReportingAt the completion of your study, you should have a list of extractable species along with their semi-quantitative concentrations to be used for your risk assessment.
Step 3 – Risk Assessment of Extractables Profile
You then need to review this data to assess if any of the extractable compounds could pose a risk to the patient or the drug product. Additional studies or resources may be necessary to help you digest the extractables data and determine an appropriate way to demonstrate safety to the regulatory bodies. For example, you can simplify your risk assessment by using West to perform an E2L™ risk-based evaluation of data generated during your extractables study. This evaluation helps you progress from the extractable stage to the leachable stage of a project.
Additionally, another way to support your extractables to leachables risk assessment, is a simulated leachable study. The purpose of a simulated leachables study is to provide you with data generated using conditions closer to actual use. The study design involves drug product or placebo in contact with packaging at conditions mimicking actual storage. The resulting data provides you with a clearer understanding of what compounds could migrate into your drug product and help narrow your toxicological assessment. You need to consider the additional time that it will take to complete simulation studies when evaluating your overall submission timeline.
A toxicological assessment is necessary to evaluate the potential risk the compounds observed are to the patient or to the drug product. E&L guidance from the Product Quality Research Institute (PQRI) suggests the use of appropriate analytical thresholds when generating extractables data by combining a concept called the Safety Concern Threshold (SCT) (which is defined as “the threshold below which a leachable would have a dose so low as to present negligible safety concerns from carcinogenic and non-carcinogenic toxic effects”) with the dosing information of the product. Compounds observed above this threshold aren’t automatic concerns but need to be evaluated on a case-by-case basis to understand the risk to the patient.
Your extractables assessment must have a clear rationale of the process your followed from identifying the materials in contact with the product and patient to the risk assessment conclusion. The conclusion should include a list of compounds to target during a leachables study and/or how the product will be monitored for leachables throughout the shelf life.
Step 4 – Demonstrate Efficacy and Safety Through Leachables Assessment
The leachables study is your final step in demonstrating compatibility between the materials of use and the final drug product. Based on the risk assessment performed in step 3, methods must be developed and validated targeting any potential problematic compounds in the final drug product at appropriate concentrations. A complete and low risk leachables study involves the use of both targeted and screening methods. Targeted methods will quantify compounds identified in the risk assessment. Non-targeted screening methods (similar to those used during extractables studies) are used to monitor the packaged drug product for unanticipated leachables throughout the shelf life of the drug product. This further supports the decisions/inclusions of targeted compounds.
Time requirements in the final step are at the highest for the entire E/L assessment process due to the need to evaluate the final packaged drug product throughout the shelf life. This includes resource heavy development and validation activities to establish targeted methods specific for the drug product under evaluation.Getting started with your E&L Assessment
Starting your E&L assessment as soon as manufacturing and packaging components are finalized is critical to meeting your timelines to show regulatory authorities that all materials used for the manufacturing, containment, and delivery are compatible with your drug product. For further details on your timeline for planning E&L assessment read West Analytical Services’ whitepaper, Is it Ever Too Soon To Start Your Extractables and Leachables Assessment?
It’s never too soon to start your extractables and leachables assessment so Contact Us today to get started!
VeriSure® and E2L™ are trademarks or registered trademarks of West Pharmaceutical Services, Inc. in the United States and other jurisdictions.




