PDA and West Sponsor Symposium on Combination Products
Do you want to better understand how to navigate the regulatory pathway to successfully bring a drug/device combination product (CP) to market? Understanding that many have this question, West, in collaboration with the Delaware Valley Chapter of PDA, sponsored the symposium (Sep 23): The Evolving Landscape for Combination Products – Defining a Regulatory Strategy. Experts from the industry and the FDA presented and discussed the latest best practices.
![]()
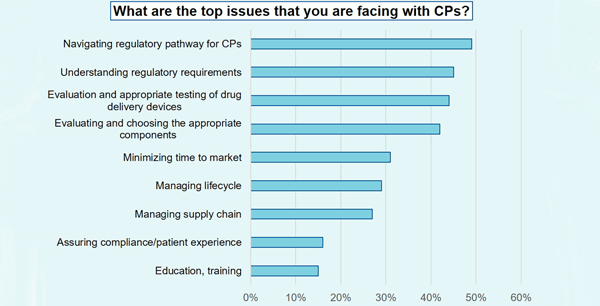
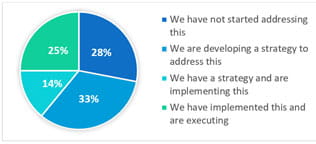
During the event, we surveyed more than 200 attendees. One of the questions was: What are the top issues that you are facing? The highest responses were:
- navigating the regulatory pathway for combination products
- understanding regulatory requirements
- evaluation and appropriate testing of drug delivery devices
From the discussion, it was clear that appropriate testing programs which are risk-based and built to inform the combination product development process are key to assuring that a development program will satisfy global regulatory agencies. There are some key themes to remember when developing any drug device combination product. They are:
- Starting with the end in mind. The development process should not be entered blindly; it must be patient-driven. This means having a good understanding of the patient, clinician, and therapeutic needs for the specific application.
- Merging aspects of Design Controls (DC) with Quality by Design (QbD).
- Building line of sight data packages to support development through regulatory approval and lifecycle management.
- Creating two-way communication channels with transparency throughout the supply chain.
Over 29% of the attendees reported struggling with lifecycle management. To provide greater support and insight, West is sponsoring a free webinar (Dec 3, 11:00 AM, EST): ICH Q12 Pharmaceutical Product Lifecycle Management and the Relevance to Combination Products. An overview and specific examples will be discussed.