Particulate Challenges
Particulates in injectable drug products continue to be an area of great interest in the industry. The main reason is the potential clinical effects on patients, which can range from emboli to inflammations to infections. There are a series of clinical risk factors that should be considered in evaluating and mitigating these challenges. These factors are route of administration, patient population, and particulate composition (i.e., number, size, and shape). All are critical inputs to understanding risk and determining corrective and preventative actions.
![]()

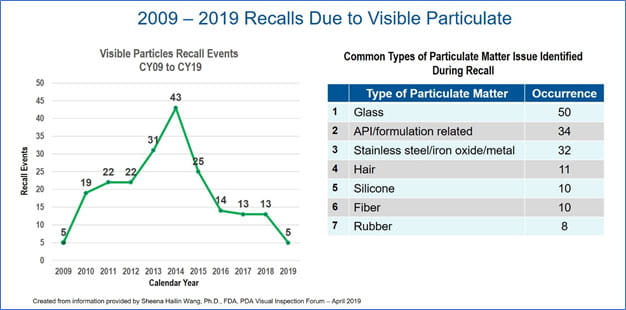
The greatest area of concern is extrinsic particulates, as these are considered foreign to the drug product. The presence of these particulates resulted in 212 recalls of injectable drug products from 2009 through early 2019 [Sheena Hailin Wang, Ph.D. (FDA/CDER/OPQ Office of New Drug Product II), Regulatory Perspective on Inspection of Injectable Products for Visible Particulates. 2019 PDA Visual Inspection Forum, Washington, DC (4/23/19). See also U.S. FDA Rep Offers Overview on Visible Particulates located at https://www.pda.org/pda-letter-portal/home]. A review of the timeline shows that recalls peaked in 2014, but since have declined. This is an improvement, but clearly there is room for further improvement.
In order to achieve further improvement, it is critical to understand testing approaches and all factors that could impact particulate formation and drug product contamination. At West, particulates are a thrust area. We have the expertise and facility not only to measure particulate levels and types precisely, but we are also actively engaged in developing new measurement methods.
For more on how West can help, contact an Account Manager or Technical Customer Support (TCS) representative.













