FlowCam®
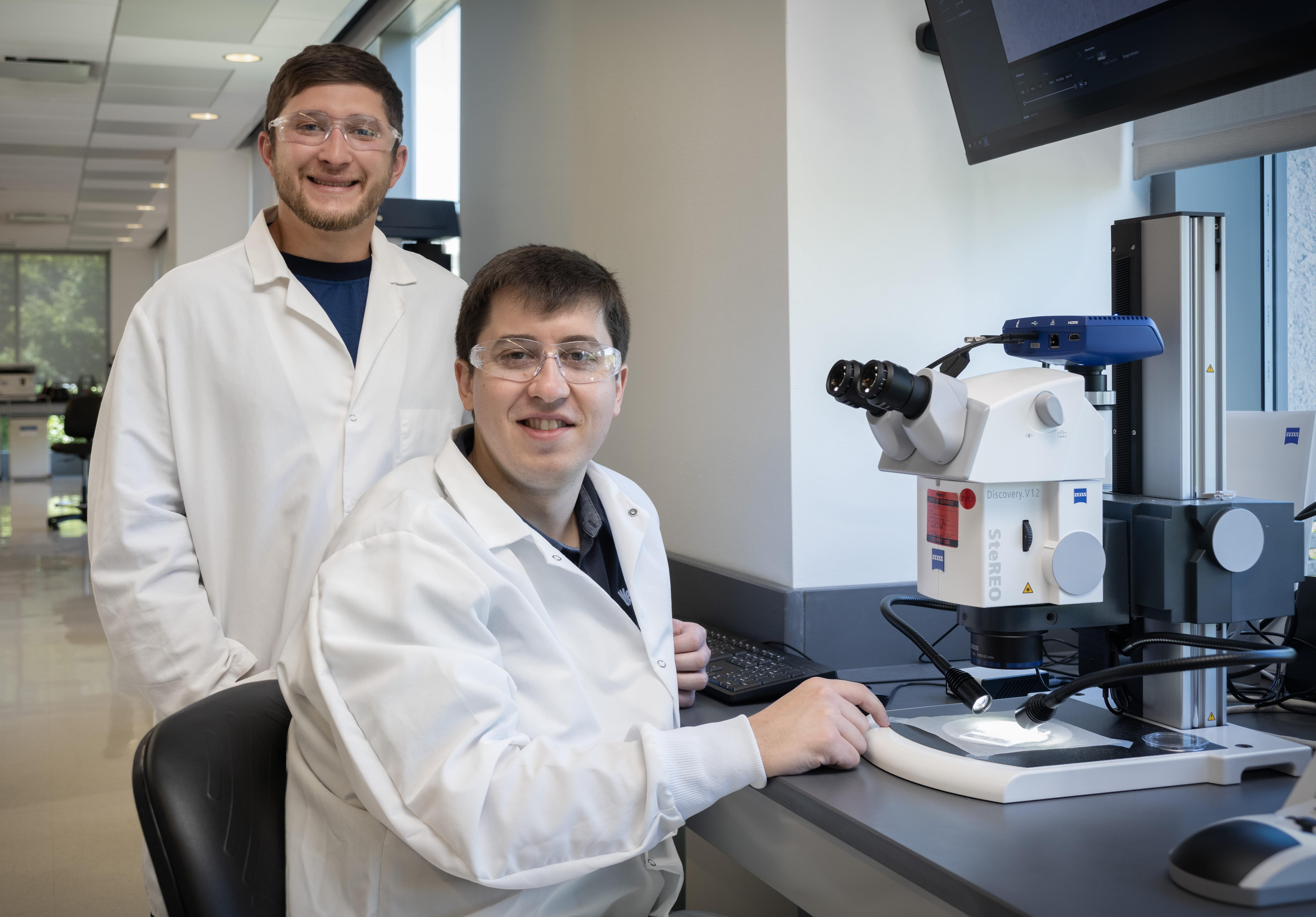
The FlowCam® 8100 (Yokogawa Fluid Imaging Technologies) is used for sub-visible particle analysis. The instrument captures digital images of particles suspended in liquid as they pass through a flow cell. Each individual particle captured in the field of view has over 40 morphological characteristics, which can be used to create image libraries for particle characterization. The instrument uses the 21 CFR Part 11 compliant software program VisualSpreadsheet® to process captured images and generate customizable reports containing particle count and size.
The FlowCam 8100 can be used to monitor particle levels in drug products and can be used as a complement to Light Obscuration (LO) analysis for early phase compatibility studies. It is especially convenient for biologics because of the small sample volume requirements - as little as 300 µL per sample analysis.
Understanding particle origin and being able to differentiate between intrinsic (related to manufacturing process), extrinsic (foreign to manufacturing process) and inherent (related to product) particles is critical to understanding the risks associated with particles in the formulation. The FlowCam, which enables particle characterization, allows for determination of particle type which can provide a deeper understanding of the particles that are present in drug products.