Industry Preparedness for Meeting the Challenges of Essential Performance Requirements
Prefilled syringes, and prefilled syringes with auto-injectors, continue to dominate the landscape. However, there has been an uptick in the evaluation and use of cartridges in on-body injector systems. The growth of these kinds of products reinforces the need for solutions to deliver larger volumes of biologic products to patients in a simplified manner. The trend from intravenous administration to subcutaneous administration helps to facilitate this patient-friendly option.

(Figure 1)
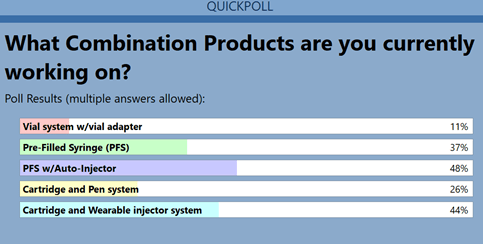
At the February 2022 West webinar,Demystifying Essential Performance Requirements (EPRs),several survey questions were posed to attendees to understand boththe industry’s knowledge of EPRs, and how prepared they are tomeet them. A question regarding what injectable combination products(CPs) are being developed revealed the following trends (Figure1):
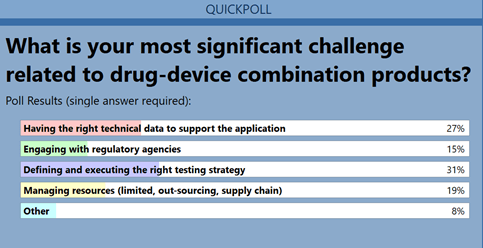
Now that these innovative CPs are available, the challenge is tomeet the needs of the regulators in order to facilitate approval foruse in the market. This challenge was the focus of the secondquestion (Figure 2). Key industry concerns include development of anappropriate testing strategy, building the correct data sets, andpreparing documentation to support the regulatory filing.
(Figure 2)
(Figure 3)
The webinar specifically focused on how to identify EPRs and relatedcontrol strategies. Having a comprehensive testing plan is criticalto regulatory submission. The underpinning of all these activitiesis a risk-based approach to understand the constituent device andfinal combination product EPRs. EPRs are defined as: a subset ofdesign controls that relate to assuring that clinical performance ofthe device itself is adequate to meet the combination productperformance intended at the point of dosing.1
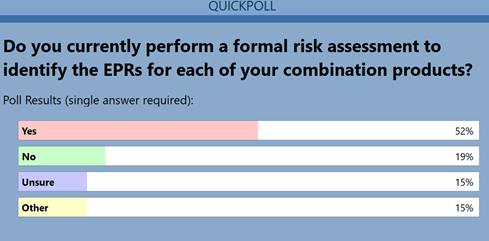
The third question revealed that approximately half the attendeesdid not utilize a risk-based process for identifying EPRs (Figure3). Not using a risk-based approach increases the potential forfailure. Challenges will arise in addressing regulatory questions onsubsequent performance testing issues if up-front work is notcompleted effectively.
EPRs and their associated control strategies are an extremelyimportant piece of the data/documentation that is assembled as partof the CP submission process. The control strategy should provide acomplete overview of all points of control relating to the CP andits constituent parts.
More details associated with EPRs and control strategies can befound by viewing this webinar atUnlocking the Mystery of Combination Product Essential PerformanceRequirements.
Reference
1. Diane Harper,Applying ICH Q12 to Combination Products. American DrugDelivery & Formulation Summit. 2019







