Lyophilized Drug Products – Selecting the Right Components
Lyophilization (freeze drying) is the process of dehydrating a material at low temperature and reduced pressure. It is used to extend the shelf life of many biologic drug products (> 400 FDA-approved); because absent water, the rates of degradative processes are reduced substantially. Essential to successful lyophilization, and subsequent rehydration, are use of proper containment and reconstitution systems.
![]()
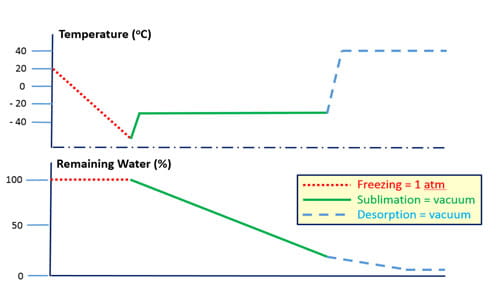
These systems, and the components that comprise them, are discussed in detail in the chapter Container and Reconstitution Systems for Lyophilized Drug Products in Lyophilization of Pharmaceuticals and Biologicals (ISBN 978-1-4939-8927-0, Humana Press, 2019) by P. McAndrew (Dir., Scientific Communications), D. Hostetler (Sr. Manager, Integrated Solutions) and F. DeGrazio (VP, Scientific Affairs & Technical Services). This chapter discusses the fundamental chemistry and physics of the lyophilization process. It also discusses (a) influence of component designs (e.g., stopper vent, vial wall thickness, vial adapter spike), and the (b) influence of component materials (e.g., thermal conductivity, water adsorption) on performance. Understanding all these factors facilitates the selection of the right components for lyophilization drug packaging systems.
For more on how West can help with containment and reconstitution systems, contact an Account Manager or Technical Customer Support representative, or visit the Knowledge Center.



