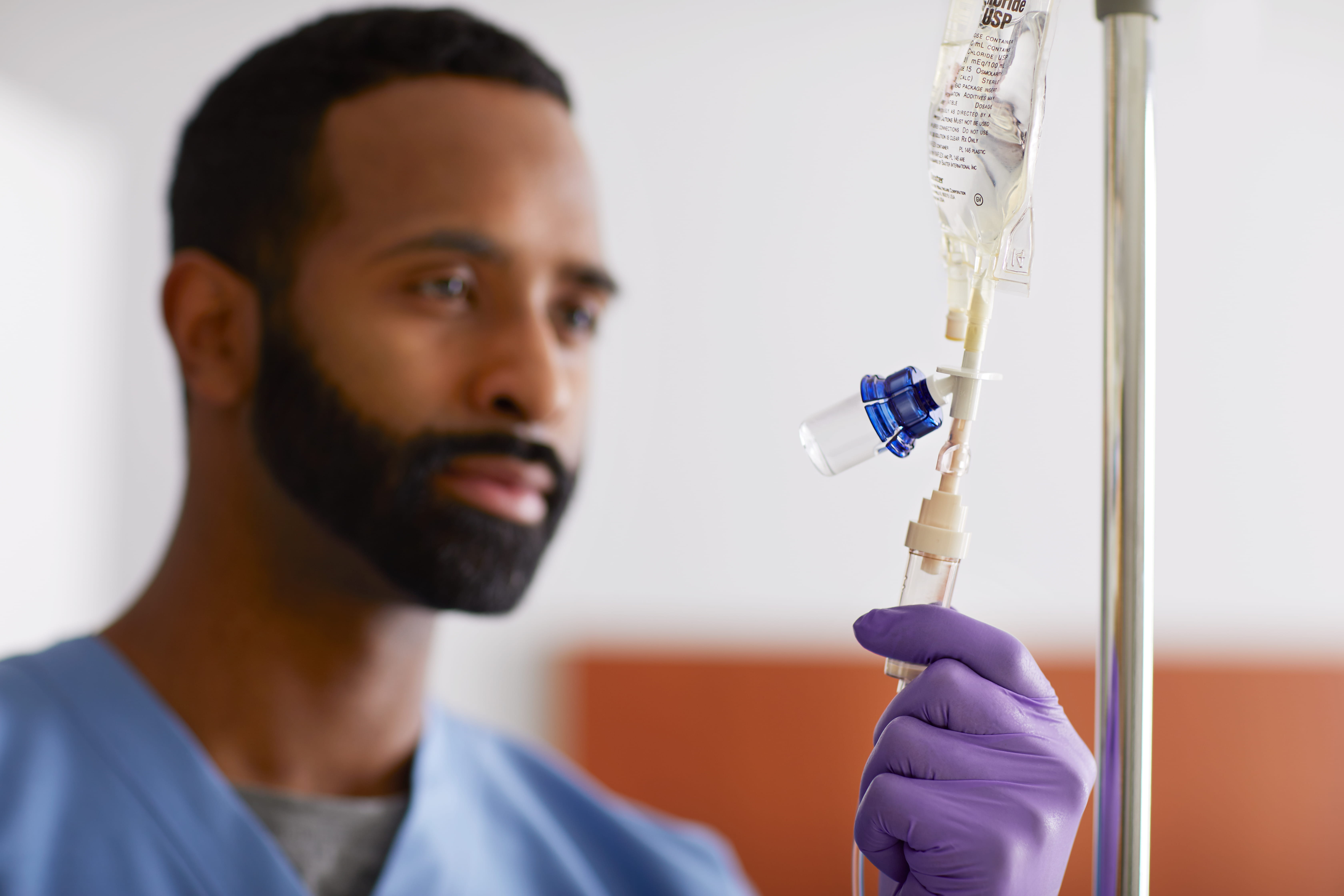
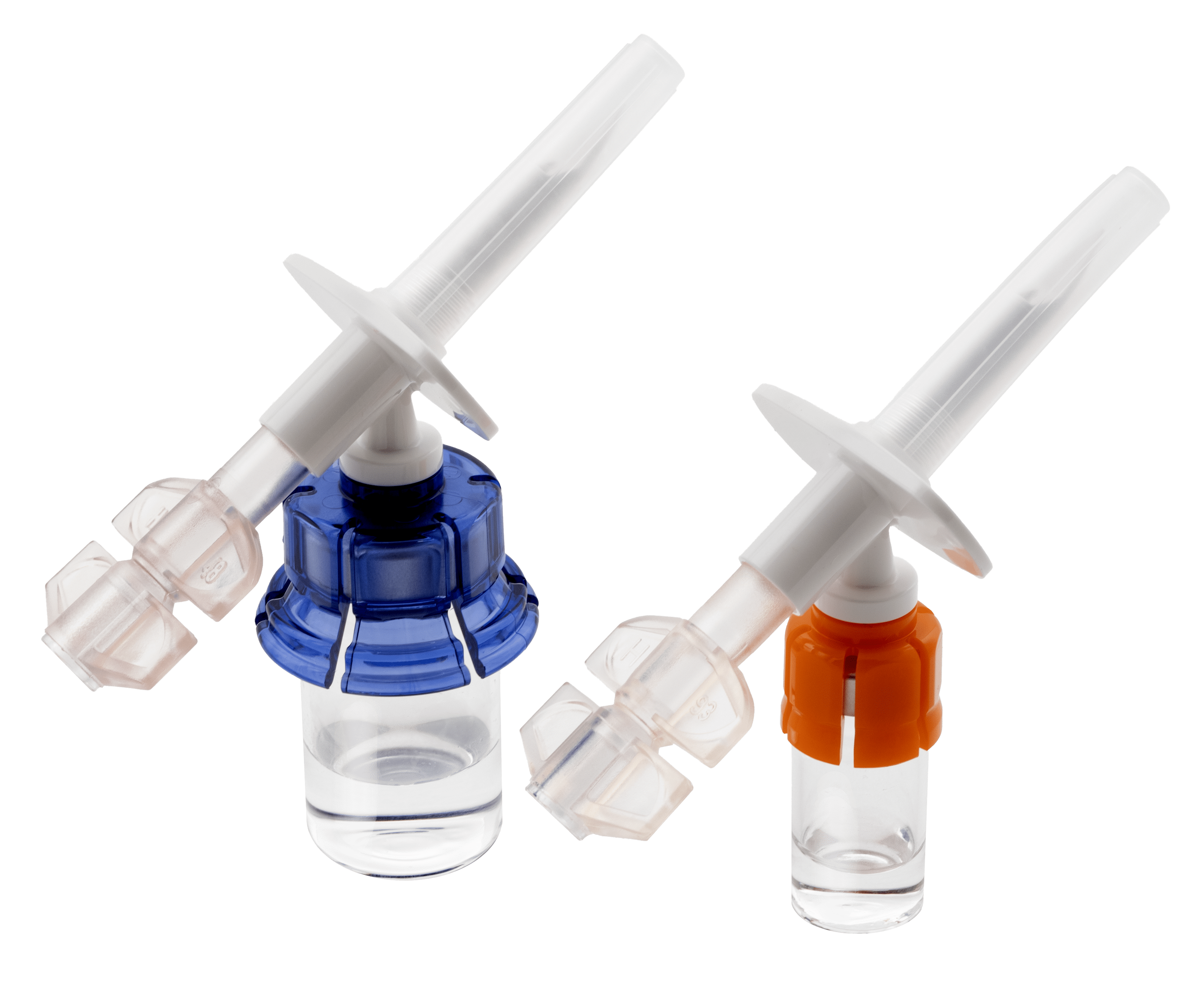
*Compatible with all manufacturers’ 50, 100, and 250 mL IV bags with ISO 8536-4 standard IV spike. **Study results yielded from a single-center retrospective analysis performed from June 2017 to July 2018 by an academic medical center using West’s older Vial2Bag DC device, which is no longer commercially available and has been replaced with devices in the Vial2Bag Advanced® admixture device portfolio.‡ The Vial2Bag Advanced® 13mm and 20mm admixture devices are 510(k) cleared by the United States Food and Drug Administration (FDA). The use of the Vial2Bag Advanced® 13mm and 20mm admixture devices should not be interpreted as modifying, extending, or superseding a drug manufacturer’s labeling recommendations for storage and expiration dating, unless otherwise limited by USP <797> compounding standards. Refer to drug manufacturer’s labeling and use instructions for recommendations, USP <797>, and applicable institution policy for shelf life and sterility information of reconstituted product and admixture device compatibility. Compatibility of the Vial2Bag Advanced® 13mm and 20mm admixture devices with all drug products has not been confirmed. Do not use the Vial2Bag Advanced® 13mm and 20mm admixture devices with lipids. Failure to follow the instructions provided may result in inadequate medication reconstitution, dilution, and/or transfer, possibly leading to overdose or underdose and/or delay in therapy. Products shown are for INFORMATION purposes only and may not be approved for marketing in specific regions. Please contact your West Pharmaceutical Services, Inc. (West) representative for product availability.West and the diamond logo is a registered trademark of West Pharmaceutical Services, Inc., in the United States and other jurisdictions.Vial2Bag Advanced® and logo and Orange and Blue Vial Adapters are registered trademarks of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.






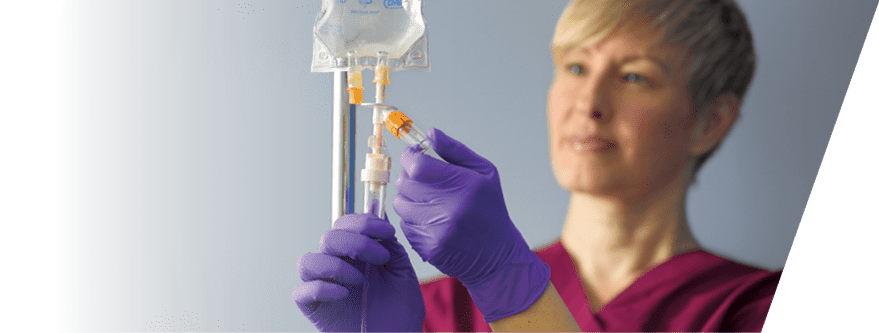


)