Best Practices to De-risk & Shorten Time to Market Through ISO 11608
The complexity of bringing a combination product, such as an on-body delivery system (OBDS), to market cannot be overstated. Changing or unclear regulations are often cited by the industry as a major source of confusion which contributes to that complexity. However, continuous incorporation of updated standards and newly issued guidance documents during the development process can reduce risk, improve patient safety, save time and headaches when the submission date ultimately approaches.
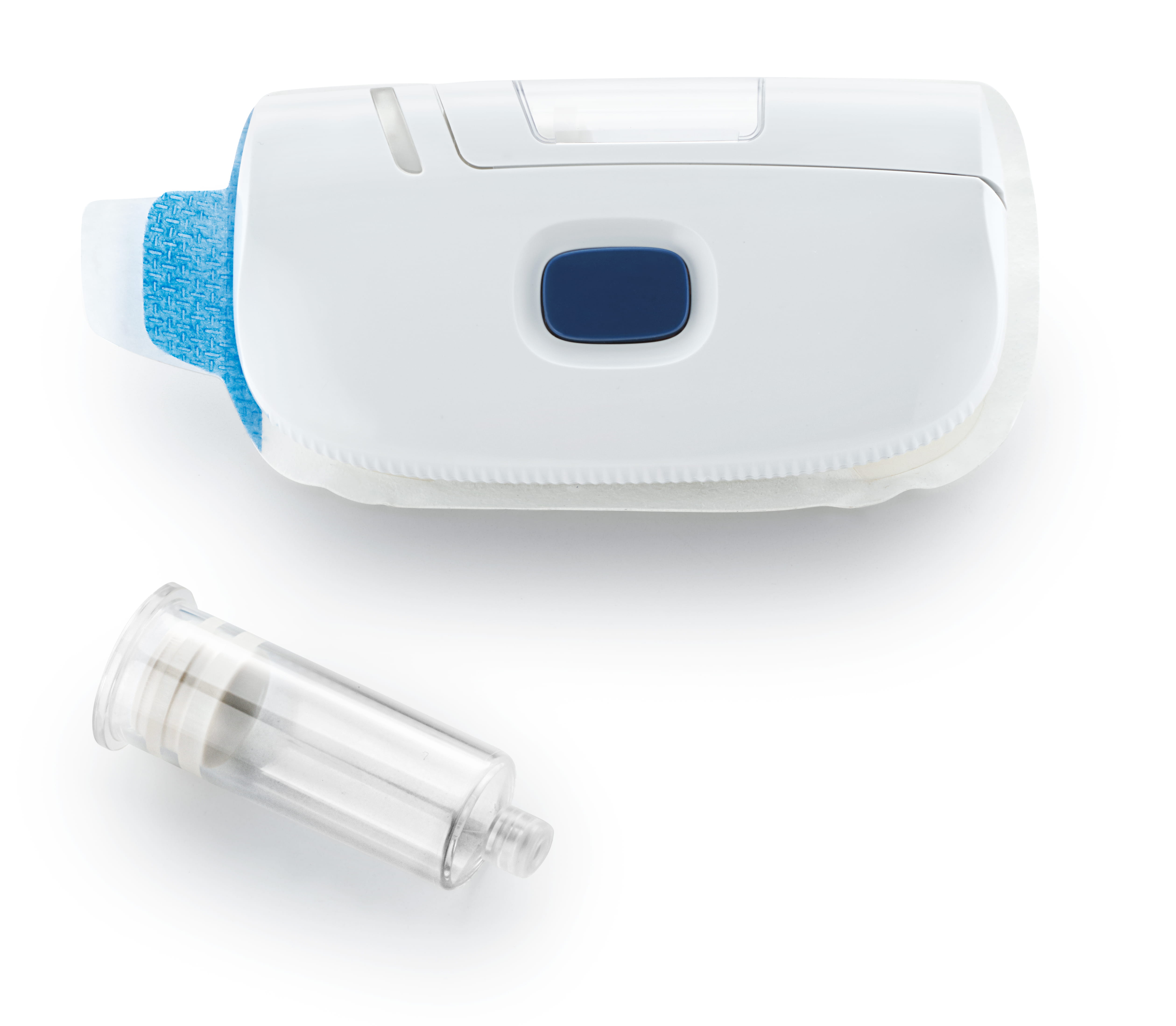
Development timelines for devices and combination products can, in some cases, progress for several years. During that time, the current thinking on the approach to safety and effectiveness continues to evolve. When these current approaches are not considered and incorporated into the development process, it can lead to the regulatory authority requiring more justification and rationale from the manufacturer which leads to delayed submissions and approvals. On the contrary, if manufacturers can continue to evaluate, assess, and implement the latest standards and guidance during the development process, they can reduce the inevitable questions that will arise during a regulatory authority review cycle. Most importantly, the continuous implementation of the latest standards and guidance ensures that patient safety remains paramount from concept to commercialization.
This is especially critical for medical devices targeted for the European Union market where there are requirements that the assessment must consider the generally accepted “state of the art”. State of the art is defined as “the most recent editions of standards published by the standardisers”1. Given this requirement, the longer the manufacturer waits to implement the latest version, the greater the challenge to claim the product is “state of the art”.
One long awaited significant change involves the revision of ISO 11608, Needle-based injection systems for medical use — Requirements and test methods: Parts 1-5, along with the addition of Part 6, which is specific to on-body delivery systems. This new standard is a result of collaboration between the ISO and major health authorities including the US FDA and was recognized as a consensus standard for on-body delivery systems on May 30th, 2022. The revisions to ISO 11608 were created in part to satisfy the increase in wearable devices, where no existing standard directly applied.
Highlights of some of the major changes are listed below:
Pump versus Injector
The 11608:2022 series addresses requirements for needle-based injection systems such as OBDS. Per the standard, to qualify as an injection system, “the time or speed employed to deliver a discrete volume would be based upon tolerability or convenience rather than clinical relevance (e.g., medication efficacy) as would be the case with insulin patch pumps or traditional infusion pumps”2. Pumps are excluded from the scope of the standard so it is critical to identify whether the OBDS would qualify as a pump or injector early in the review process.
Reorganization of the chapters and “Roadmap”
Overarching content that was previously covered in subsequent parts has now been centralized in Part 1 - Needle Based Injection Systems. Part 1 is viewed as the parent chapter for which all products covered in the scope of the series should comply. The roadmap is included to guide the user on how to use the subsequent chapters based on how the product is classified. For OBDS specifically, manufacturers will need to use the roadmap as outline requirements, starting with Part 1, Part 6 – On Body Delivery Systems and any other applicable chapters in the series.
Introduction of primary functions
The concept of primary functions is new to the 11608 series. Primary functions include functions or operations where, if they failed to operate or perform according to specifications, would impact the dose from being delivered accurately via the correct route and/or create unacceptable harm to the patient. Determination of primary functions should be on “top level” characteristics that are functions or operations of the device. Characterization of non-functions, such as biocompatibility, pyrogenicity, and others are still important and required to be addressed in the standard but do not qualify as primary functions. Once primary functions are identified, those functions are subject to significant preconditioning prior to evaluation to ensure they meet all required specifications.
Requirements for robust particulate evaluation
Particulate, although addressed in previous revisions, is much more comprehensive in the latest version. Evaluation of both visible and subvisible particulate is required. Methodology is specified in the standard along with compendial and ISO references. For visible particulate, references are made to USP <790> and ISO 8536-4, along with USP <382> to evaluate fragmentation of elastomeric components such as lined seals. For subvisible particulate, USP <788> is referenced along with ISO 8536-4.
Particulate is a focus area for the regulatory authorities due to the potential of significant patient risk and one of the main sources of recalls globally. Therefore, it is critical for manufacturers to have a holistic and robust approach to particulate evaluation.
Expansion of Means of Attachment – On-Body Delivery Systems
The interface between the body and the device is critical to ensure the function is not interrupted by the device being displaced from the body during use. Therefore, the requirements for evaluation of the adhesion to the device and from the device to the body is significantly expanded in the new revision. Manufacturers need to identify performance that includes exposure to “expected fluids” that may be present on the body during normal use. These include (but are not limited to) water, deodorant, lotions, and perspiration. The manufacturer should ensure that the means of attachment is adequate to maintain a reliable medicinal product pathway while also not creating harm to the patient through tissue trauma.
In addition, device specific requirements exist throughout the chapter as well as cross referencing across the chapters. Therefore, manufacturers should be sure to review the entire 11608 series to ensure all requirements are taken into consideration for their development program.
Overall, an OBDS is only as good as the manufacturers’ ability to prove, with clear objective evidence, that the device is safe and effective. Therefore, understanding and incorporating the latest applicable standards and guidance into supporting documentation allows manufacturers to benefit from already agreed upon approaches, rather than trying to justify a different path. This mindset of continuous incorporation of the latest established thinking improves regulatory sustainability of the OBDS along with improving patient safety.
Interested in learning more about ISO 11608 and best practices to de-risk and shorten time to market for wearable injectors? Click here to register for our webinar next week.
Important product information at: https://www.westpharma.com/products/self-injection-platforms/smartdose/smartdose-10
References1MDCG 2021-5, Guidance on standardisation for medical devices, April 2021
2ISO 11608-1:2022, Needle-based injection systems for medical use — Requirements and test methods — Part 1: Needle-based injection systems, April 2022




