Maintaining superior viral vector recovery in cell and gene therapy applications by using Daikyo Crystal Zenith® vials
West partnered with SK Pharmteco to evaluate the impact of vial material of construction on the stability of adeno-associated virus (AAV) serotypes under varying stress and storage conditions associated with cell and gene therapy applications. AAV8 and AAV9 were chosen as representative serotypes, with respective titers in a common buffer formulation (10mM Tris-HCl, 3.5mM NaCl, 8.5% Trehalose, 0.06% PS80, pH 7.2). This was evaluated across three 2mL vial types : Daikyo Crystal Zenith® (CZ), and two commercially available borosilicate glass containers. All containers were sealed with West 13mm Serum NovaPure® 1358 4023/50G bromobutyl stoppers and filled with 0.35mL fill volume.
To simulate viral vector recovery in cell and gene therapy applications, the vial combinations were tested across five challenge conditions:
Table 1: Challenge conditions used to simulate Cell & Gene Therapy Applications
Study Condition | Timepoint | Rationale |
|---|
| T0 | T0 | Starting point |
| Long Term -80°C | 3 months | Common recommended storage condition. |
| Accelerated at 2-8°C | 1 month | Refrigeration, temporary storage at site |
| 2 months |
| Stress at 25°C | 1 week | Room temperature storage, clinical ambient conditions |
| 2 weeks |
| 3 weeks |
| Freeze Thaw | 1 cycle* | Mimic of handling during manufacturing. Potential structural damage to proteins and DNA |
| 3 cycle* |
| 5 cycles |
| Agitation (250RPM) | 72 hours | Mimic of handling during manufacturing and shipping. Potential protein unfolding and degradation due to shear. |
| Dry Ice Exposure (Naked Vial Packaged in Cryobox & Biohazard Plastic Bag) | 5 days | Mimic of handling during shipping. Potential for CO2 ingress to impact quality |
*Samples allocated for 1 and 2 cycle of freeze-thaw will be recycled for additional stability timepoints for the -80°C condition.
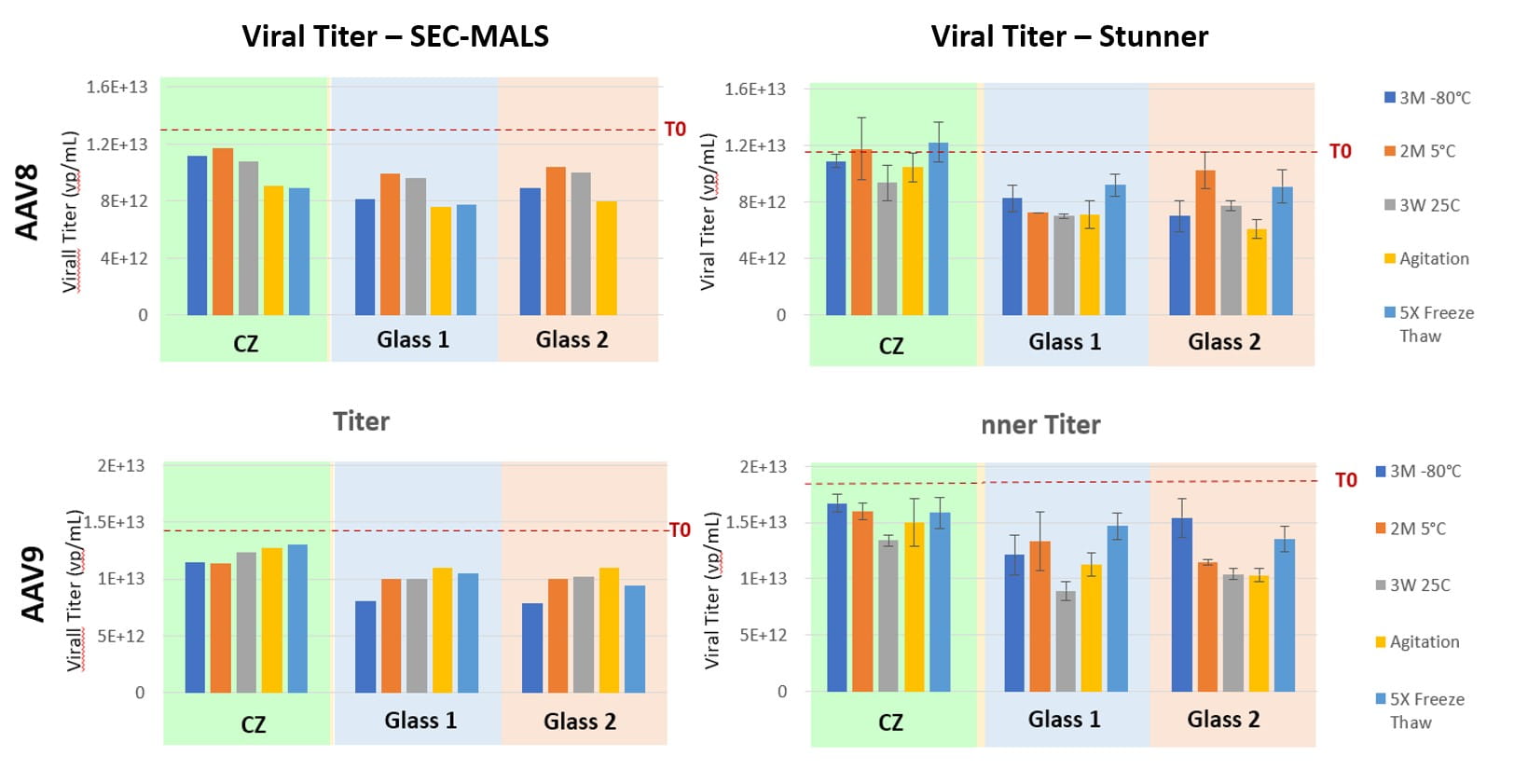
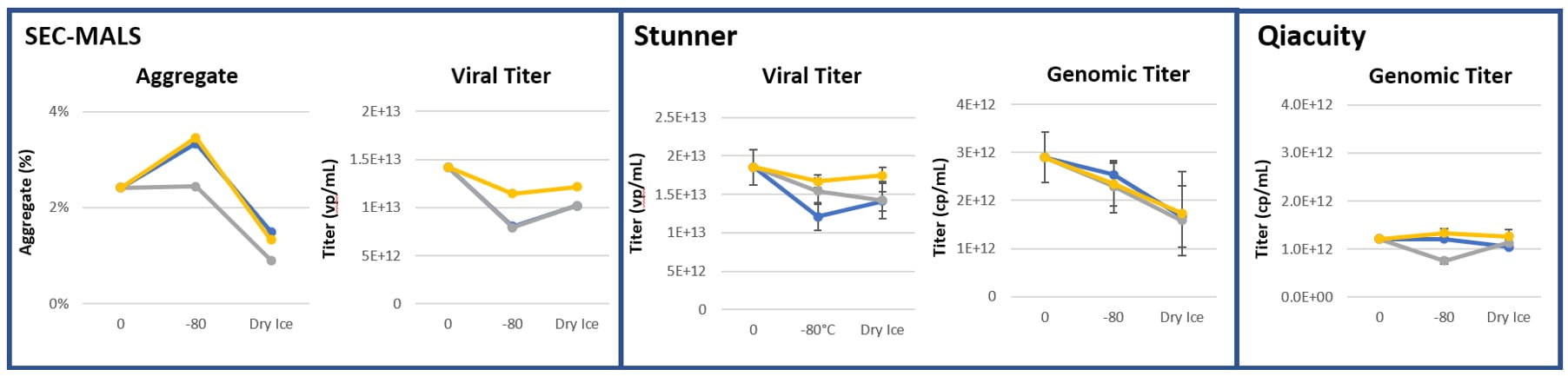
A variety of orthogonal methods were used to quantify viral titer, including dPCR (QIAcuity, Qiagen instrument); high-speed UV/Vis spectroscopy with static and dynamic light scattering (Stunner, Unchained Labs equipment); and SEC-MALS (Size-exclusion chromatography with multi-angle light scattering, Wyatt equipment). Figure 1 demonstrates a higher viral titer shown in CZ containers compared the borosilicate glass comparators across measurement methods and challenge conditions.
Figure 1: Viral Titer measurements (n=3)
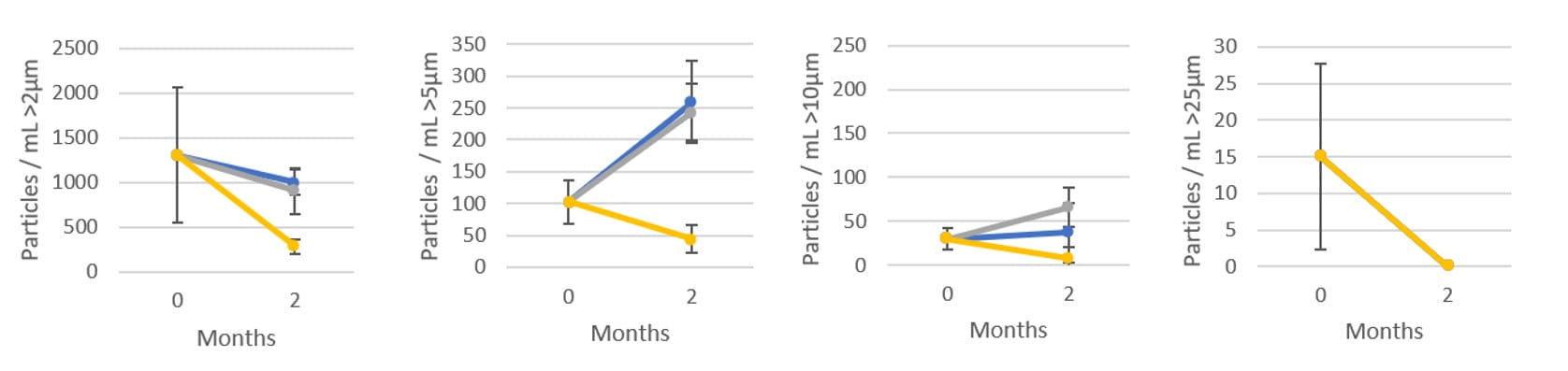
Subvisible genomic particles were analyzed using Halo Labs Aura GT and all conditions were again tested in triplicate. Figures 2-4 demonstrate a lower propensity of particle agglomeration in CZ compared to the glass vials.

Figure 2: Aura GT particle measurement of AAV9 at 2 months @ 2-8°C (n=3)
Note: All data for >25um is equal and thus hidden under the same line
Figure 3: Aura GT particle measurement of AAV8 at 3 months @ -80°C , and 1 week naked vial dry ice storage (n=3)

Figure 4: Aura GT particle measurement of AAV9 at 3 months @ -80°C, and 1 week naked vial dry ice storage (n=3)

After storage for 2 months at 2-8°C, 3 months at -80°C and 1 week on dry ice, the CZ vial showed lower particle counts than their glass vial counterparts for particles up to and including >5µm, while maintaining comparable measurements with larger particles.
Most of the stress conditions produced no other significant changes in product quality in all vials tested, shown by Figures 5-12 below. A BMG Labtech Nephelostar was used to measure turbidity.
Figure 5: AAV8, 2 months at 2-8°C (n=3)
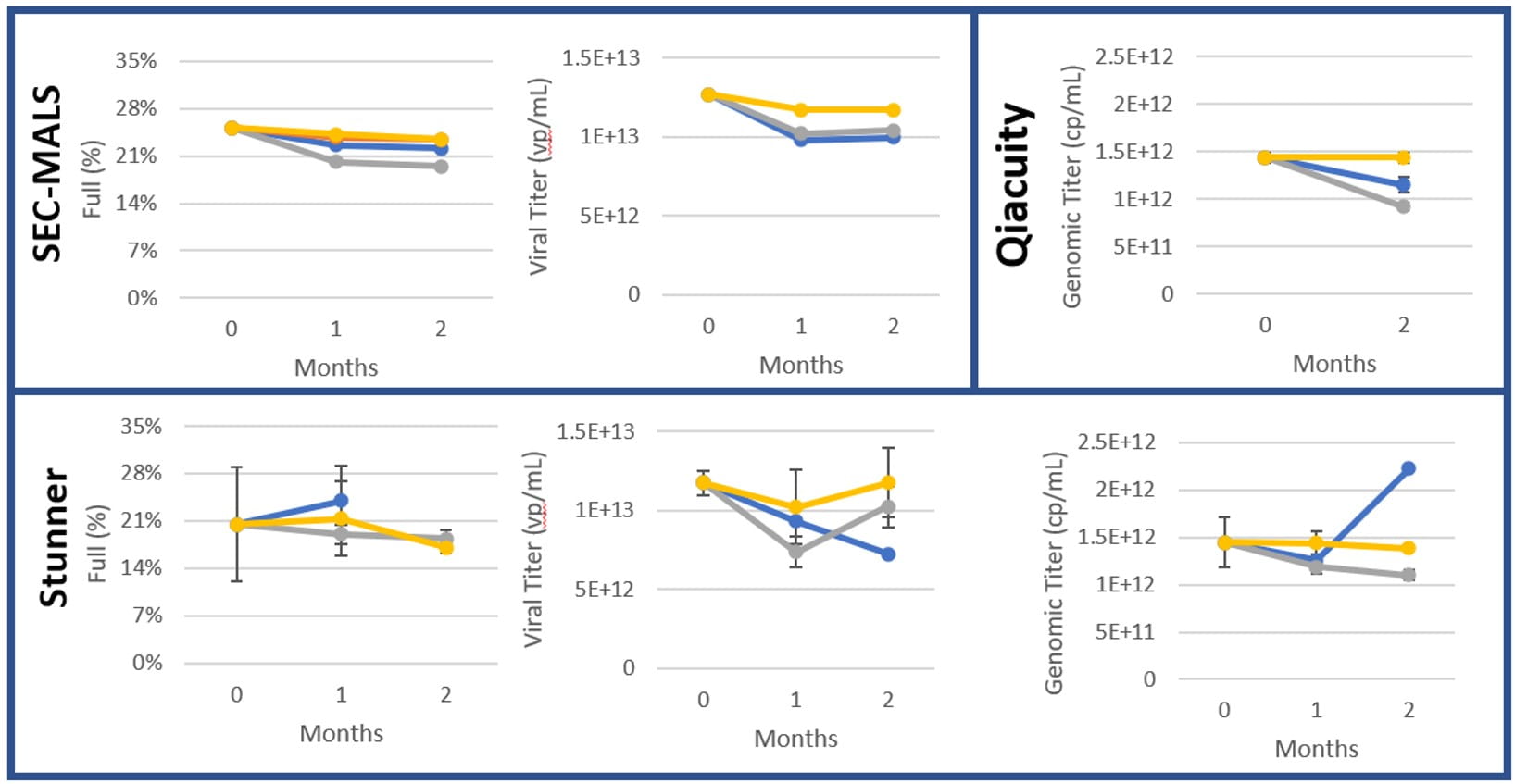
Figure 6: AAV9, 2 months at 2-8°C (n=3)
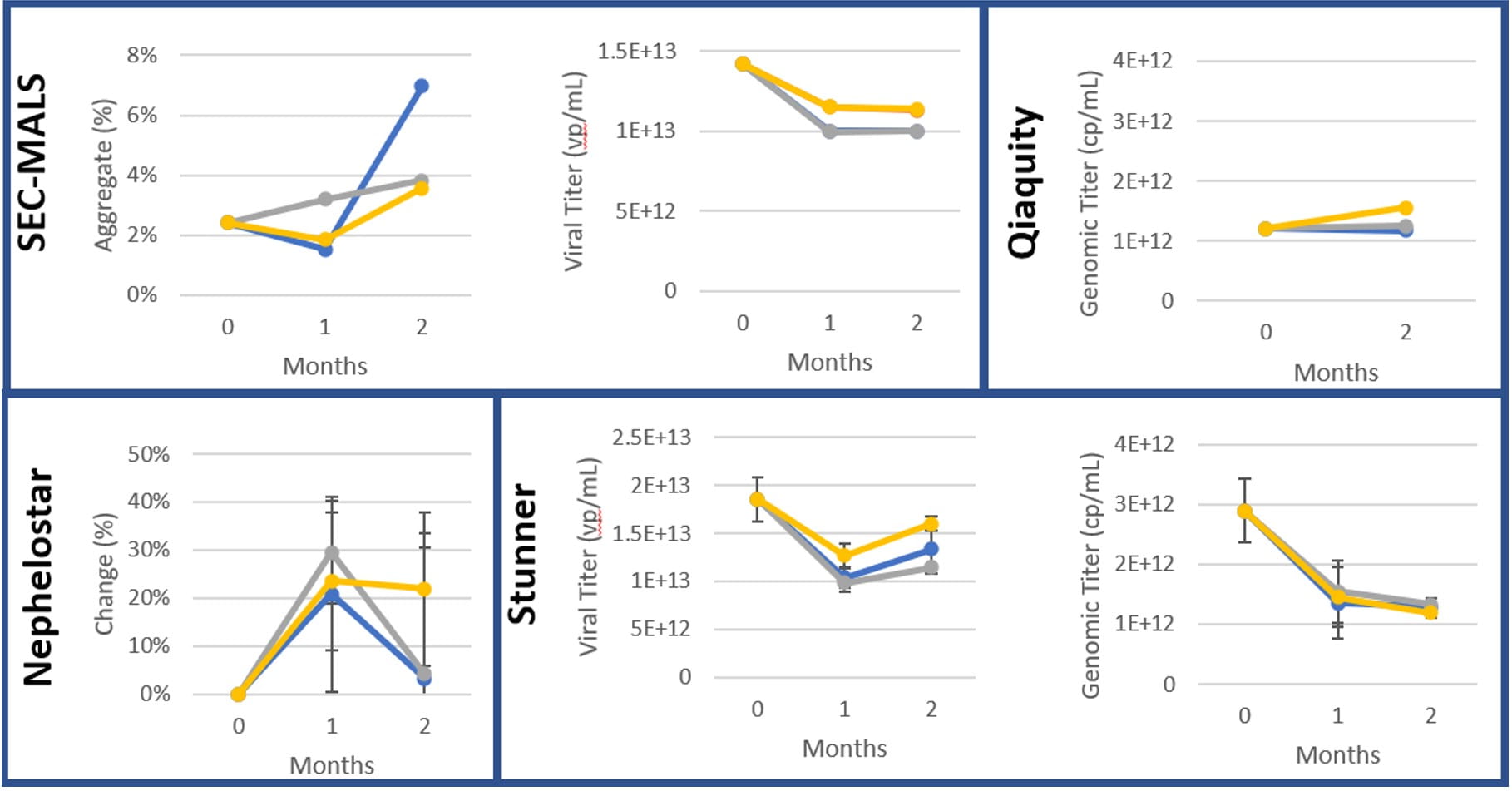
Figure 7: AAV8, Ambient storage at 25°C (n=3)
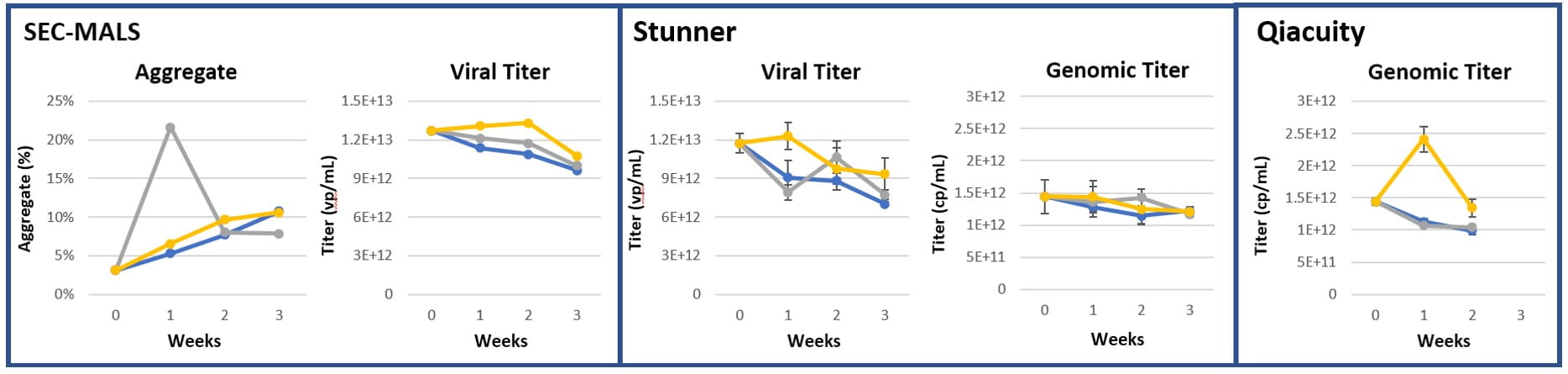
Figure 8: AAV9, Ambient storage at 25°C (n=3)

Figure 9: AAV8, Agitation (250 RPM) (n=3)
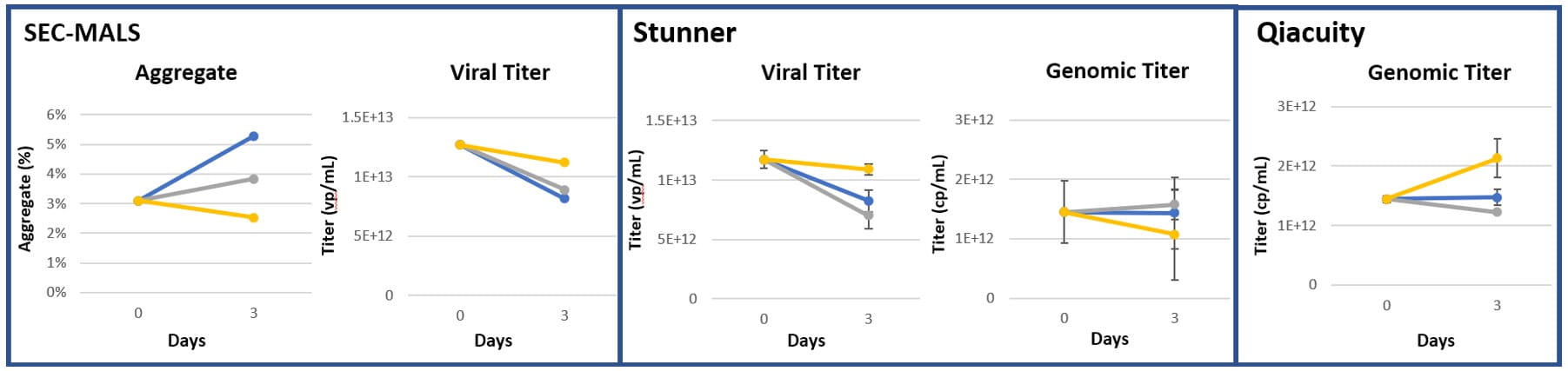
Figure 10: AAV9, Agitation (250 RPM) (n=3)
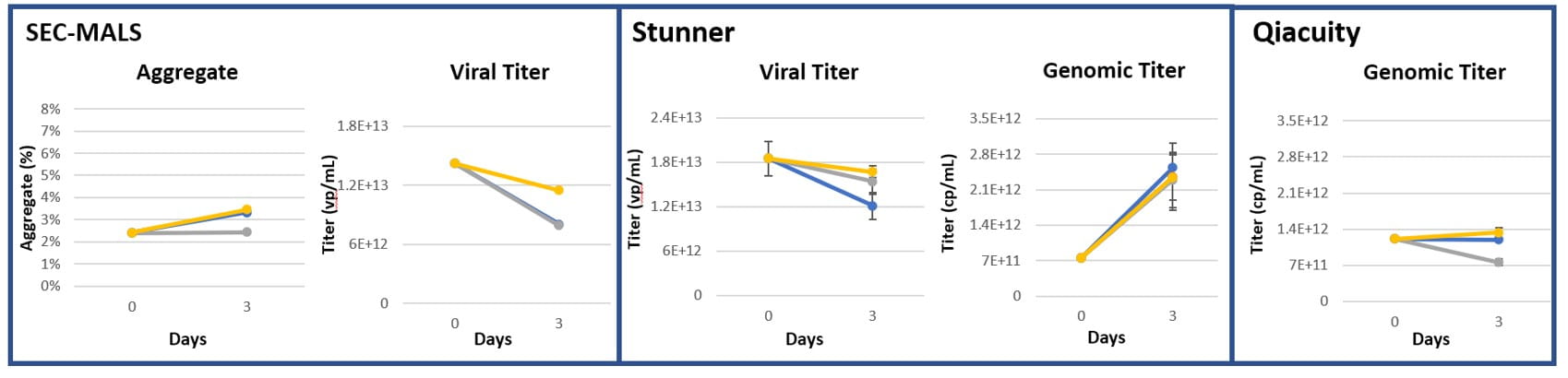
Figure 11: AAV8, 3 months at -80°C and 1 week naked vial dry ice storage (n=3)
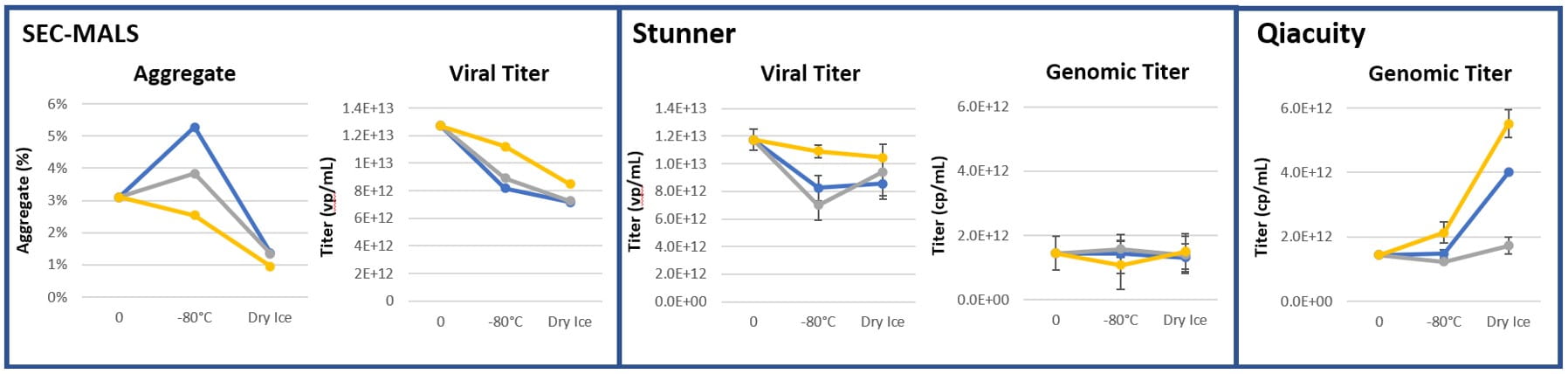
Figure 12: AAV9, 3 months at -80°C and 1 week naked vial dry ice storage (n=3)
In summary, the Daikyo CZ vial demonstrated a consistent increase in viral vector recovery compared to borosilicate glass vials across all challenge conditions, while no negative effects in product quality were observed due to container storage in the Daikyo CZ vials ,
AAV9 was shown to be less stable than AAV8, and shear stress appeared to be a pronounced stress condition for AAV9. Furthermore, aggregation is the prevalent degradation mechanism for handling stresses.
The data shows that the CZ vials may comparatively improve viral vector recovery across common storage and handling conditions used in cell and gene therapy applications. It is imperative that the drug maker evaluate these simulated conditions with their drug-specific materials and relevant environments to ensure optimal results for the intended therapy. For more information on Daikyo Crystal Zenith® vials click here.
West would like to thank SK Pharmteco (formerly Center for Breakthrough Medicines) for their collaboration in running this study.
West and the diamond logo and NovaPure are registered trademarks of West Pharmaceutical Services, Inc. in the United States and other jurisdictions.
Crystal Zenith is a trademark of Daikyo Seiko, Ltd.
All other trademarks are the properties of their respective owners.
Crystal Zenith technology is licensed from Daikyo Seiko, Ltd.
Copyright ©2024 West Pharmaceutical Services, Inc. All rights reserved.

















