NovaPure® 3mL Cartridge Components: Quality by Design (QbD) Principles Mitigate Risks Associated with Biologic Drugs Packaging and Delivery Systems
Globally, the market for biologic drugs is growing significantly, due to their proven ability to treat major chronic diseases, in particular those found among an increasingly aging population. Cartridge systems are very important in delivering these biologic drugs; they can be used in autoinjectors, pen injectors, and wearable devices. Since biologics drugs are very sensitive, they require highest-quality cartridge components.
![]()
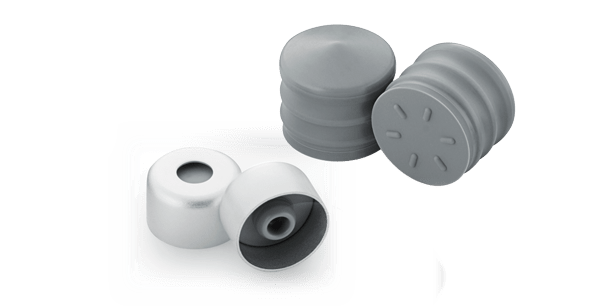
Anticipating both technical and market needs, West has designed, and manufactures, components compatible with 3mL ISO glass cartridge barrels, namely NovaPure® cartridge components comprising 3mL cartridge plungers and 8mm lined seals. These components were developed based on Quality-by-Design (QbD) principles. The QbD approach is a systematic, science-based approach to manufacturing that supports the highest levels of process understanding. This includes identification and monitoring of critical process parameters, targeted improvements to optimize critical processes, continuous improvement based on statistical analysis, and a documented product knowledge database.
Key features of NovaPure 3mL cartridge components are:
NovaPure 3mL cartridge components help mitigate risks associated with biologic drug packaging and delivery components, and help to achieve the primary objective of patient safety. For more information click here or contact an Account Manager or Technical Customer Support (TCS) representative.
NovaPure® and FluroTec® are registered trademarks of West Pharmaceutical Services, Inc., in the United States and other jurisdictions.
FluroTec® and B2-coating technologies are licensed from Daikyo Seiko, Ltd.



