Why FluroTec® Barrier Film for Lyophilization Stoppers
Lyophilization (freeze drying) is the process of dehydrating a material at low temperature and reduced pressure. It is used to extend the shelf life of many biologic drug products. Absent water, degradation processes are greatly reduced. In any primary package system (i.e., vial, elastomer stopper, seal) for a lyophilized drug product, of utmost concern are leachables and volatile organic compounds that may migrate from the elastomer stopper into the lyophilized drug product, creating a risk to patient safety.
![]()
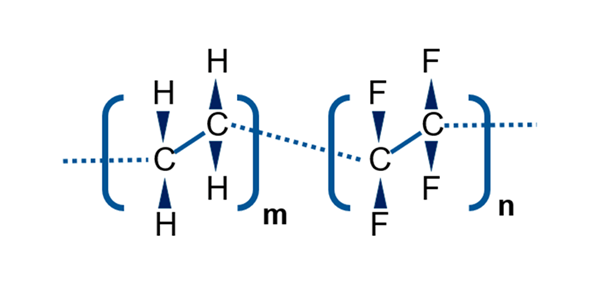
A way to mitigate this concern is with elastomer stoppers with FluroTec® barrier film. The FluroTec film, which is based on the co-polymer poly(ethylene tetrafluoroethylene), is laminated onto the elastomer stopper. It serves as an effective barrier between the drug product and elastomer, reducing the risk of leachables and volatile organic compounds from contacting the drug product. This has been demonstrated experimentally and was discussed in detail in a recent white paper by West scientists, Elastomer Stoppers with FluroTec® Film: The Right Choice for SARS-CoV-2 Vaccines, which considers the importance of FluroTec barrier films for SARS-CoV-2 vaccines.
For more information on West products and services, contact an Account Manager or Technical Customer Service (TCS) representative.
FluroTec® is a registered trademark of West Pharmaceutical Services, Inc., in the United States and other jurisdictions.
FluroTec® technology is licensed from Daikyo Seiko, Ltd.



