Rx Only
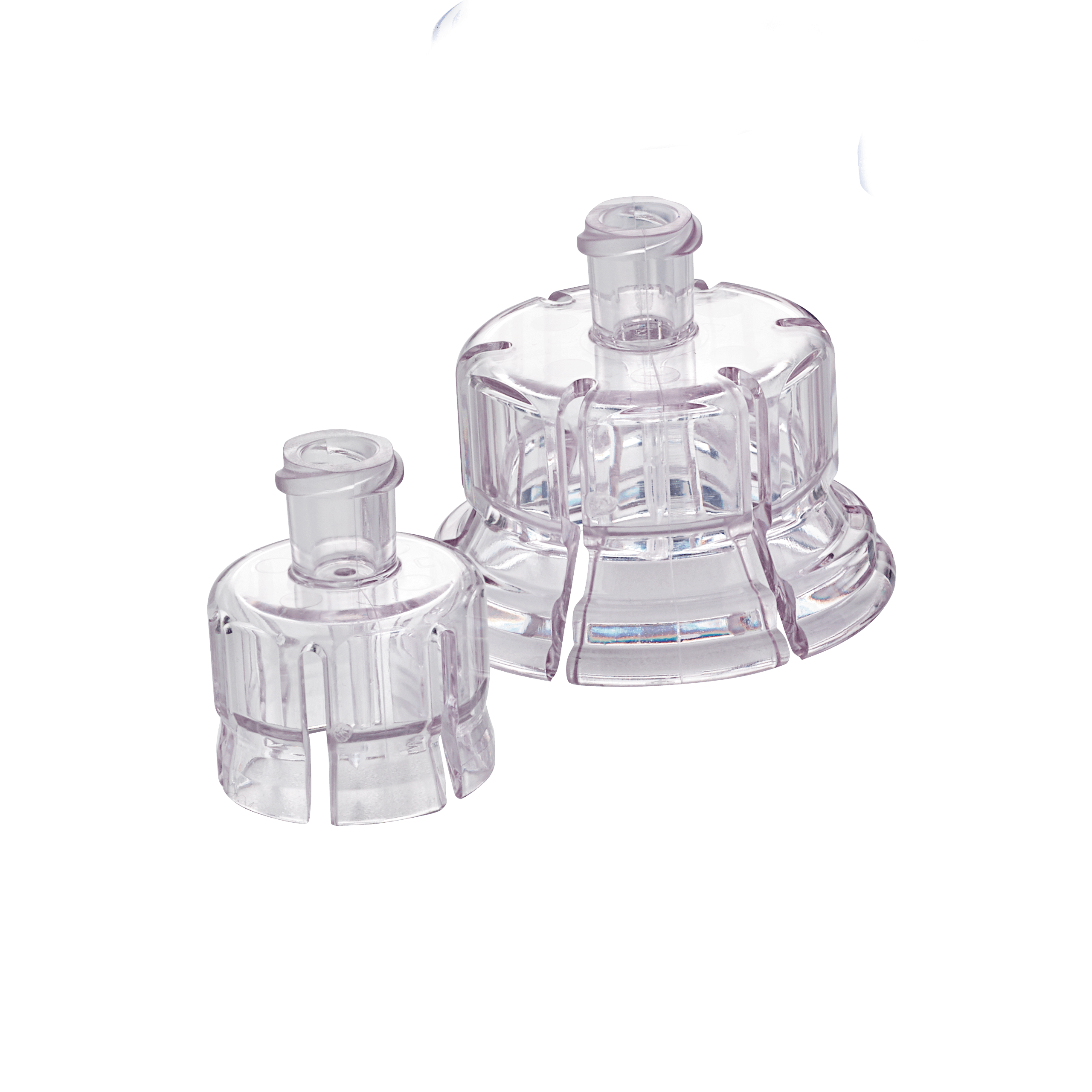
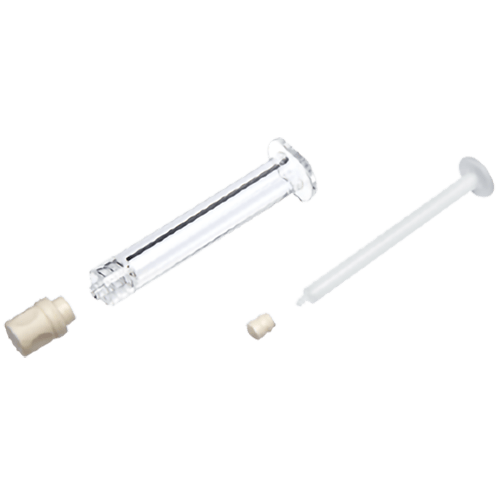
The MixJect® transfer device is 510(k) cleared by the United States Food and Drug Administration and carries the CE mark (0344). Products are shown for INFORMATION purposes only and may not be approved for marketing in specific regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. The MixJect® transfer device is configurable and may not be suitable for use with all drugs. Refer to drug manufacturer's labeling and use instructions for device configuration compatibility. The device may contain a pre-attached needle containing cobalt. Injection site pain, irritation, discomfort, bruising, and/or erythema may occur. Failure to follow product instructions for use may result in compromised sterility; contamination; leakage (including possible exposure to medication); and/or inadequate medication reconstitution, transfer, and/or dosing. Product misuse could potentially lead to needlestick injury, user and/or patient exposure to pathogens or infection, and/or suboptimal or delayed therapy. Please contact your West Pharmaceutical Services, inc. (West) representative for product availability.
Mix2Vial®, MixJect®, and Vial Adapter™ are trademarks or registered trademarks of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.
Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd.
Crystal Zenith® technology is licensed from Daikyo Seiko, Ltd.



)
)

)
)