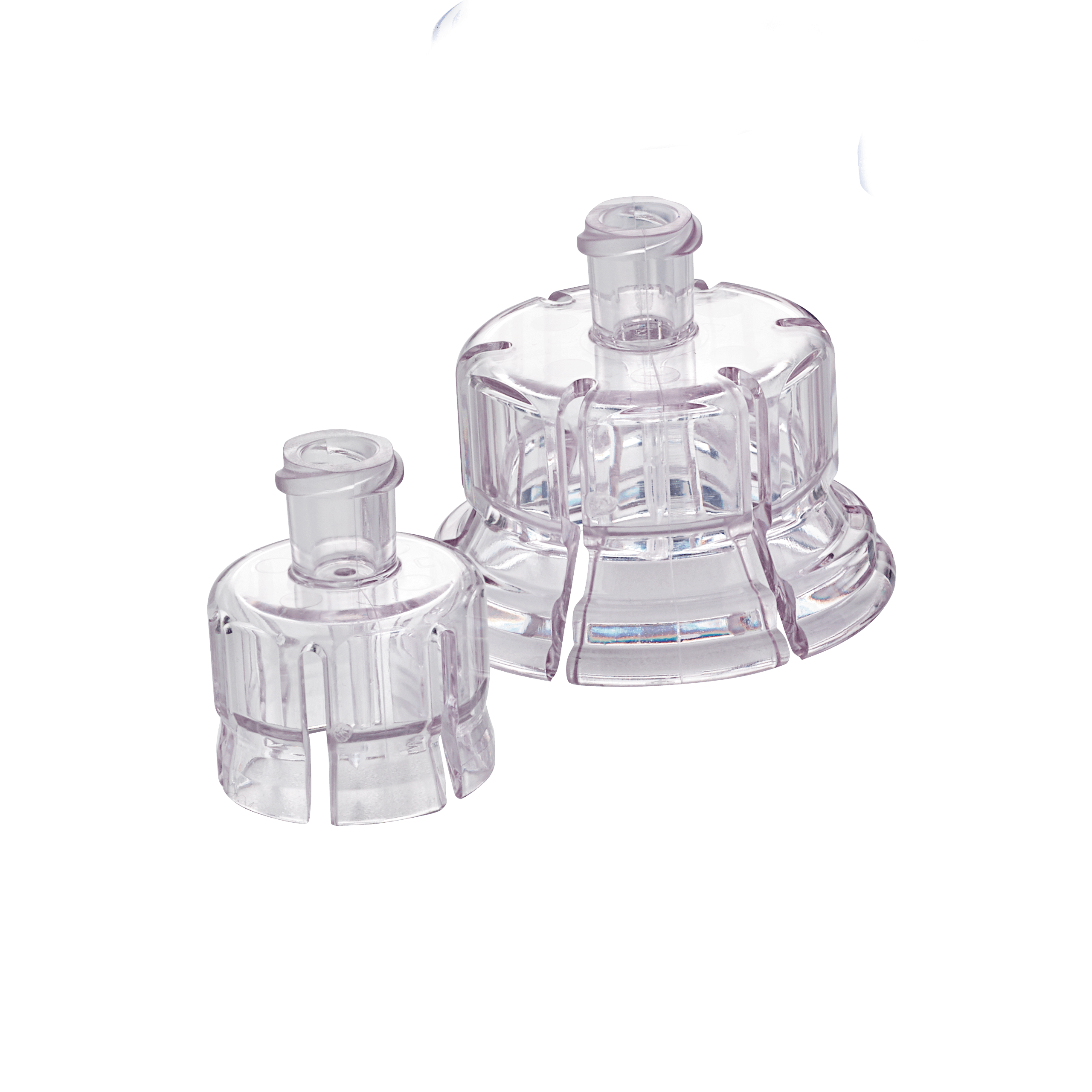
The Mix2Vial Transfer Device is:
- Easy to use
- Designed for lyophilized drugs with fixed volume diluent
Performance Options:
Components are available to work with 20mm stoppers. Puncturing the elastomeric closure of a drug vial is achieved by means of an integral spike.
The Mix2Vial® transfer device is 510(k) cleared by the United States Food and Drug Administration and carries the CE mark (0344). Products are shown for INFORMATION purposes only and may not be approved for marketing in specific regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. Failure to follow product instructions for use may result in loss of sterility; contamination; inadequate transfer, dilution, and/or withdrawal; and/or medication dosing. Product misuse could potentially lead to user and/or patient infection, suboptimal therapy, and/or delay in therapy. Please contact your West Pharmaceutical Services, Inc. (West) representative for product availability.
Mix2Vial Transfer Device Indication, Safety and Warnings



)
)


)
)
