Improve Speed to Market by Mitigating Vial and Stopper Incompatibility
Selection of the appropriate primary packaging system is essential to delivering a high quality, safe, and stable drug product to patients. The primary packaging components which make up the containment system are selected on a range of factors, from chemical compatibility with the drug product ingredients to dimensional fit when mating packaging components. Optimized fit among mating packaging components is essential for flawless fill-finish processing.
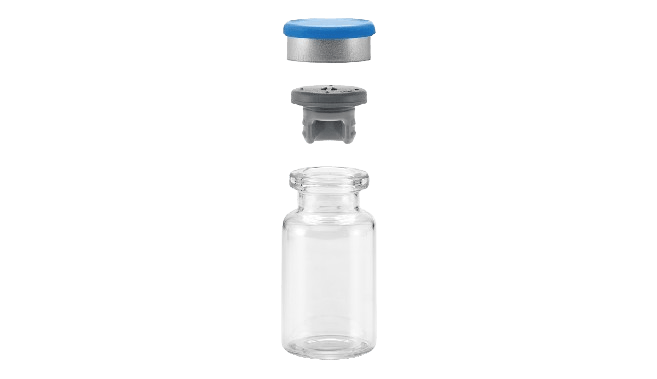
Glass vials are typically available in three neck types, namely US blowback, EU blowback, and non-blowback. Blowback features on vials, and no-pop ring features on stoppers, reduce the risk of stopper pop-up phenomena during fill-finish processing prior to vial crimping. Considering the surge in demand for primary packaging components due to the Covid-19 pandemic, many affordable medicine manufacturers were forced to switch vial types to ensure continuity of supply. But many were faced with using a vial with a different neck type.
Understanding the pressure that puts on manufacturers, West conducted two, two-year studies that indicate for a properly-assembled vial containment system, good container closure integrity performance can be achieved with stoppers of a given elastomer, independent of configuration/size or vial style. For 4031/45 Gray in particular, across stopper size/configuration and vial style, no performance variation was observed. This suggests strongly that the chosen 4031/45 Gray stoppers should perform well independent of vial style, with proper system assembly.
Mapping risks to container closure integrity up front and early in development, alongside selecting components demonstrated to mating together well, inevitably help affordable medicine manufacturers mitigate risk and delays in drug development.
Another such way to reduce delays is to minimize lead times associated with component supply, which can often be lengthy, and force additional delays and supply constraints onto manufacturers. All such delays in bringing the molecule from the laboratory to the patient, can directly impact the success of the drug product, as market share uptake is closely tied with speed to market for affordable medicine manufacturers.

Selected stoppers in modern, elastomer formulation 4031/45 Gray are now available within the AccelTRA offer, ensuring supply of the right quality stoppers to meet the market need and they are available for quick delivery from stock. Alongside a robust extractables package to truncate testing timelines, these stoppers provide extensive functionality data, and timely regulatory support.
For more information on the AccelTRA Offer click here or contact an Account Manager or Technical Customer Support (TCS) representative.
AccelTRA® is a registered trademark of West Pharmaceutical Services, Inc., in the United States and other jurisdictions.





