What is Cold?
Temperature sensitive drug products, such as biologics or insulin, often require cold storage. But what, exactly is “cold”? We know it when we feel it, but often when one person is shivering, another is quite comfortable. Like humans, drug products may have specific tolerances to cold that depend upon the product.
![]()
Cold, when it comes to drug products, is defined as any temperature not exceeding 8°C. A common temperature range for drugs requiring cold storage is anywhere from 2°C to 8°C. Additional parameters for cold storage may be required, including light and air quality levels such as humidity, or carbon dioxide or oxygen levels.
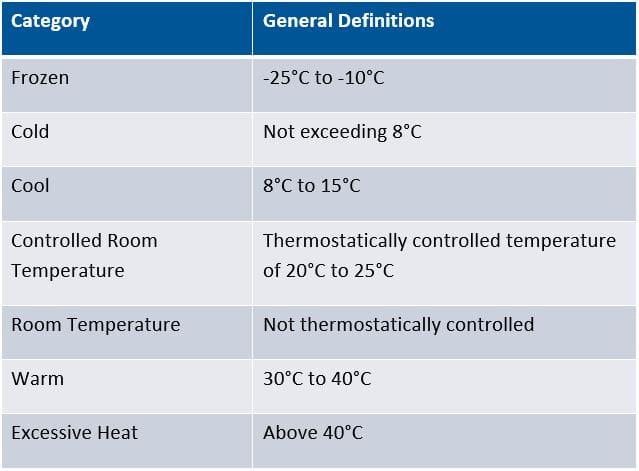
The following chart shows the common temperature nomenclature used by the US Pharmacopeia:
To ensure that the cold chain is maintained, pharmaceutical developers and their contract manufacturing partners must use controlled environments and monitor those environments closely. Refrigerated warehouses, trucks, containers and ships, as well as insulated shipping containers or specialized packaging are used to protect drugs during transit.
West Contract Manufacturing offers pharma customers integrated device assembly, drug handling and cold chain solutions that meet regulatory and FDA guidance for temperature sensitive drugs. This helps to ensure that drugs reach the patients who need them without being rendered useless by poor environmental factors.
Contact us today to determine how West Contract Manufacturing can support cold chain solutions.










