Looking for Ways to Reduce End of Line Rejects Significantly
In today’s manufacturing processes, defects such as loose and embedded foreign matter may cause end of line rejects of drug products—potentially leading to a loss of product or even market recalls. At the same time, regulatory authorities are becoming increasingly more stringent and continually lowering acceptable levels of particulate matter.
![]()
The West Envision™ verification system can help with both of these challenges, as it enhances the quality of Westar® ready-to-sterilize pharmaceutical components by significantly reducing adhered and embedded particulate found in primary pharmaceutical packaging components. Envision verification is also a tool to effectively reduce cosmetic defects that are of special concern in some markets like Japan. Furthermore, if a customer has special concerns, West can complete a standard defect library to catalogue findings.
The West Envision verification system uses automatic, program-controlled vision inspection technology to inspect all surfaces of elastomeric components. Envision inspected products assist in reducing the total cost of goods by minimizing the risk of drug product rejection because of visible particulates and closure defects.
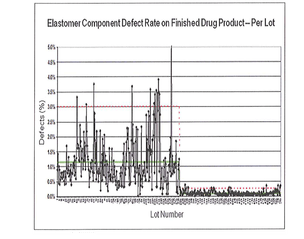
Compared to other inspection methods, Envision verification allows for 100% inspection of all articles from solid to complex deep cavities passing through the machine. Therefore, you can be assured that every Envision inspected item packed for delivery to your facility meets enhanced quality specifications by statistical means.
West Envision components help reduce lot-to-lot variability in stoppers and syringe plungers. This leads to increased product yield, because the number of drug product rejects is minimized. Ultimately, the customer’s operational efficiency increases because of optimized throughput.
The West Envision verification system is an optional tool that can be used with Westar RS and Westar RU quality, and is an inherent part of West’s NovaPure® components.




